Liposomal cisplatin combined with paclitaxel versus cisplatin and paclitaxel in non-small-cell lung cancer: a randomized phase III multicenter trial
- PMID: 20439345
- PMCID: PMC2962260
- DOI: 10.1093/annonc/mdq234
Liposomal cisplatin combined with paclitaxel versus cisplatin and paclitaxel in non-small-cell lung cancer: a randomized phase III multicenter trial
Abstract
Background: Liposomal cisplatin is a new formulation developed to reduce the systemic toxicity of cisplatin while simultaneously improving the targeting of the drug to the primary tumor and to metastases by increasing circulation time in the body fluids and tissues. The primary objectives were to determine nephrotoxicity, gastrointestinal side-effects, peripheral neuropathy and hematological toxicity and secondary objectives were to determine the response rate, time to tumor progression (TTP) and survival.
Patients and methods: Two hundred and thirty-six chemotherapy-naive patients with inoperable non-small-cell lung cancer were randomly allocated to receive either 200 mg/m² of liposomal cisplatin and 135 mg/m² paclitaxel (arm A) or 75 mg/m² cisplatin and 135 mg/m² paclitaxel (arm B), once every 2 weeks on an outpatient basis. Two hundred and twenty-nine patients were assessable for toxicity, response rate and survival. Nine treatment cycles were planned.
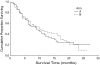
Results: Arm A patients showed statistically significant lower nephrotoxicity, grade 3 and 4 leucopenia, grade 2 and 3 neuropathy, nausea, vomiting and fatigue. There was no significant difference in median and overall survival and TTP between the two arms; median survival was 9 and 10 months in arms A and B, respectively, and TTP was 6.5 and 6 months in arms A and B, respectively.
Conclusions: Liposomal cisplatin in combination with paclitaxel has been shown to be much less toxic than the original cisplatin combined with paclitaxel. Nephrotoxicity in particular was negligible after liposomal cisplatin administration. TTP and survival were similar in both treatment arms.
Figures
References
-
- Pfister DG, Johnson DH, Azzoli CG, et al. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guidelines: update 2003. J Clin Oncol. 2004;22:330–353. - PubMed
-
- Scaglioti GV, DeMarinis F, Rinaldi M, et al. for the Italian Lung Cancer Project. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol. 2002;20:4285–4291. - PubMed
-
- Kelly K, Crowley T, Bunn PA, Jr., et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non-small-cell lung cancer: a Southwest Oncology Group trial. J Clin Oncol. 2001;19:3210–3218. - PubMed
-
- Le Chevalier T, Scaglioti G, Natale R, et al. Efficacy of gemcitabine plus platinum chemotherapy compared with other platinum containing regimens in advanced non-small-cell lung cancer. A meta-analysis of survival outcomes. Lung Cancer. 2005;47:69–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical