Removal of Spindly from microtubule-attached kinetochores controls spindle checkpoint silencing in human cells
- PMID: 20439434
- PMCID: PMC2861194
- DOI: 10.1101/gad.1886810
Removal of Spindly from microtubule-attached kinetochores controls spindle checkpoint silencing in human cells
Abstract
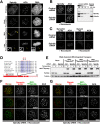
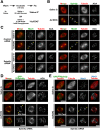
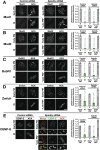
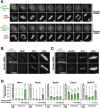
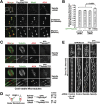
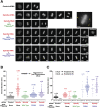
The spindle checkpoint generates a "wait anaphase" signal at unattached kinetochores to prevent premature anaphase onset. Kinetochore-localized dynein is thought to silence the checkpoint by transporting checkpoint proteins from microtubule-attached kinetochores to spindle poles. Throughout metazoans, dynein recruitment to kinetochores requires the protein Spindly. Here, we identify a conserved motif in Spindly that is essential for kinetochore targeting of dynein. Spindly motif mutants, expressed following depletion of endogenous Spindly, target normally to kinetochores but prevent dynein recruitment. Spindly depletion and Spindly motif mutants, despite their similar effects on kinetochore dynein, have opposite consequences on chromosome alignment and checkpoint silencing. Spindly depletion delays chromosome alignment, but Spindly motif mutants ameliorate this defect, indicating that Spindly has a dynein recruitment-independent role in alignment. In Spindly depletions, the checkpoint is silenced following delayed alignment by a kinetochore dynein-independent mechanism. In contrast, Spindly motif mutants are retained on microtubule-attached kinetochores along with checkpoint proteins, resulting in persistent checkpoint signaling. Thus, dynein-mediated removal of Spindly from microtubule-attached kinetochores, rather than poleward transport per se, is the critical reaction in checkpoint silencing. In the absence of Spindly, a second mechanism silences the checkpoint; this mechanism is likely evolutionarily ancient, as fungi and higher plants lack kinetochore dynein.
Figures
References
-
- Abrieu A, Magnaghi-Jaulin L, Kahana JA, Peter M, Castro A, Vigneron S, Lorca T, Cleveland DW, Labbe JC 2001. Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint. Cell 106: 83–93 - PubMed
-
- Buffin E, Lefebvre C, Huang J, Gagou ME, Karess R 2005. Recruitment of Mad2 to the kinetochore requires the Rod/Zw10 complex. Curr Biol 15: 856–861 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
