First analysis of a bacterial collagen-binding protein with collagen Toolkits: promiscuous binding of YadA to collagens may explain how YadA interferes with host processes
- PMID: 20439473
- PMCID: PMC2897373
- DOI: 10.1128/IAI.01057-09
First analysis of a bacterial collagen-binding protein with collagen Toolkits: promiscuous binding of YadA to collagens may explain how YadA interferes with host processes
Abstract
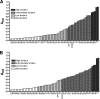
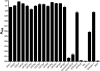
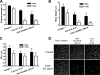
The Yersinia adhesin YadA mediates the adhesion of the human enteropathogen Yersinia enterocolitica to collagens and other components of the extracellular matrix. Though YadA has been proposed to bind to a specific site in collagens, the exact binding determinants for YadA in native collagen have not previously been elucidated. We investigated the binding of YadA to collagen Toolkits, which are libraries of triple-helical peptides spanning the sequences of type II and III human collagens. YadA bound to many of them, in particular to peptides rich in hydroxyproline but with few charged residues. We were able to block the binding of YadA to collagen type IV with the triple-helical peptide (Pro-Hyp-Gly)(10), suggesting that the same site in YadA binds to triple-helical regions in network-forming collagens as well. We showed that a single Gly-Pro-Hyp triplet in a triple-helical peptide was sufficient to support YadA binding, but more than six triplets were required to form a tight YadA binding site. This is significantly longer than the case for eukaryotic collagen-binding proteins. YadA-expressing bacteria bound promiscuously to Toolkit peptides. Promiscuous binding could be advantageous for pathogenicity in Y. enterocolitica and, indeed, for other pathogenic bacteria. Many of the tightly binding peptides are also targets for eukaryotic collagen-binding proteins, and YadA was able to inhibit the interaction between selected Toolkit peptides and platelets. This leads to the intriguing possibility that YadA may interfere in vivo with host processes mediated by endogenous collagen-binding proteins.
Figures



Similar articles
-
Characterization of the binding region for the Yersinia enterocolitica adhesin YadA on types I and II collagen.Arthritis Rheum. 1995 Sep;38(9):1283-9. doi: 10.1002/art.1780380917. Arthritis Rheum. 1995. PMID: 7575724
-
The Yersinia adhesin YadA binds to a collagenous triple-helical conformation but without sequence specificity.Protein Eng Des Sel. 2008 Aug;21(8):475-84. doi: 10.1093/protein/gzn025. Epub 2008 May 7. Protein Eng Des Sel. 2008. PMID: 18467342 Free PMC article.
-
Functional mapping of the Yersinia enterocolitica adhesin YadA. Identification Of eight NSVAIG - S motifs in the amino-terminal half of the protein involved in collagen binding.Mol Microbiol. 2000 Jul;37(1):192-206. doi: 10.1046/j.1365-2958.2000.01992.x. Mol Microbiol. 2000. PMID: 10931316
-
Yersinia adhesin A (YadA)--beauty & beast.Int J Med Microbiol. 2015 Feb;305(2):252-8. doi: 10.1016/j.ijmm.2014.12.008. Epub 2014 Dec 24. Int J Med Microbiol. 2015. PMID: 25604505 Review.
-
YadA, the multifaceted Yersinia adhesin.Int J Med Microbiol. 2001 Aug;291(3):209-18. doi: 10.1078/1438-4221-00119. Int J Med Microbiol. 2001. PMID: 11554561 Review.
Cited by
-
The Love and Hate Relationship between T5SS and Other Secretion Systems in Bacteria.Int J Mol Sci. 2023 Dec 24;25(1):281. doi: 10.3390/ijms25010281. Int J Mol Sci. 2023. PMID: 38203452 Free PMC article.
-
Coaggregation properties of trimeric autotransporter adhesins.Microbiologyopen. 2020 Oct;9(10):e1109. doi: 10.1002/mbo3.1109. Epub 2020 Aug 30. Microbiologyopen. 2020. PMID: 32864901 Free PMC article.
-
Type V secretion: mechanism(s) of autotransport through the bacterial outer membrane.Philos Trans R Soc Lond B Biol Sci. 2012 Apr 19;367(1592):1088-101. doi: 10.1098/rstb.2011.0208. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 22411980 Free PMC article. Review.
-
Trimeric autotransporter adhesin-dependent adherence of Bartonella henselae, Bartonella quintana, and Yersinia enterocolitica to matrix components and endothelial cells under static and dynamic flow conditions.Infect Immun. 2011 Jul;79(7):2544-53. doi: 10.1128/IAI.01309-10. Epub 2011 May 2. Infect Immun. 2011. PMID: 21536788 Free PMC article.
-
The effect of purity upon the triple-helical stability of collagenous peptides.Biomaterials. 2011 Sep;32(27):6621-32. doi: 10.1016/j.biomaterials.2011.05.025. Epub 2011 Jun 12. Biomaterials. 2011. PMID: 21663955 Free PMC article.
References
-
- Black, S. D., and D. R. Mould. 1991. Development of hydrophobicity parameters to analyze proteins which bear post- or cotranslational modifications. Anal. Biochem. 193:72-82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

