Complement receptor 1 expression on mouse erythrocytes mediates clearance of Streptococcus pneumoniae by immune adherence
- PMID: 20439480
- PMCID: PMC2897369
- DOI: 10.1128/IAI.01263-09
Complement receptor 1 expression on mouse erythrocytes mediates clearance of Streptococcus pneumoniae by immune adherence
Abstract
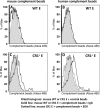
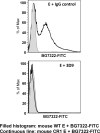
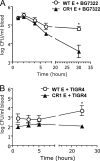
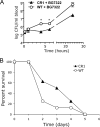
Complement-containing immune complexes can be presented to phagocytes by human erythrocytes bearing complement receptor 1 (CR1). Although this has long been assumed to be a mechanism by which humans are able to protect themselves from "extracellular" bacteria such as pneumococci, there is little direct evidence. In these studies we have investigated this question by comparing results for erythrocytes from transgenic mice expressing human CR1 on their erythrocytes to the results for wild-type mouse erythrocytes that do not express CR1. We demonstrate that human CR1 expression on murine erythrocytes allows immune adherence to beads opsonized with either mouse or human serum as a source of complement. The role of CR1 in immune adherence was supported by studies showing that it was blocked by the addition of antibody to human CR1. Furthermore, human CR1 expression enhances the immune adherence of opsonized pneumococci to erythrocytes in vitro, and the pneumococci attached to erythrocytes via CR1 can be transferred in vitro to live macrophages. Even more importantly, we observed that if complement-opsonized pneumococci are injected intravenously with CR1(+) mouse erythrocytes into wild-type mice (after a short in vitro incubation), they are cleared faster than opsonized pneumococci similarly injected with wild-type mouse erythrocytes. Finally, we have shown that the intravenous (i.v.) injection of pneumococci into CR1(+) mice also results in more rapid blood clearance than in wild-type mice. These data support that immune adherence via CR1 on erythrocytes likely plays an important role in the clearance of opsonized bacteria from human blood.
Figures
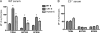
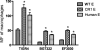
References
-
- Aaberge, I. S., J. Eng., G. Lermark, and M. Lovik. 1995. Virulence of Streptococcus pneumoniae in mice: a standardized method for preparation and frozen storage of the experimental bacterial inoculum. Microb. Pathog. 18:141-152. - PubMed
-
- Birmingham, D. J., and L. A. Hebert. 2001. CR1 and CR1-like: the primate immune adherence receptors. Immunol. Rev. 180:100-111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

