Histone H2B C-terminal helix mediates trans-histone H3K4 methylation independent of H2B ubiquitination
- PMID: 20439497
- PMCID: PMC2897581
- DOI: 10.1128/MCB.01008-09
Histone H2B C-terminal helix mediates trans-histone H3K4 methylation independent of H2B ubiquitination
Abstract
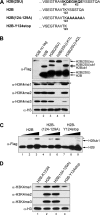
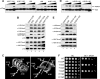
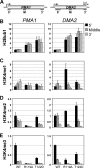
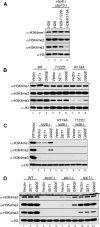
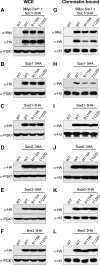
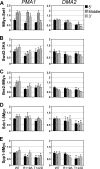
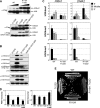
The trans-histone regulatory cross talk between H2BK123 ubiquitination (H2Bub1) and H3K4 and H3K79 methylation is not fully understood. In this study, we report that the residues arginine 119 and threonine 122 in the H2B C-terminal helix are important for transcription and cell growth and play a direct role in controlling H2Bub1 and H3K4 methylation. These residues modulate H2Bub1 levels by controlling the chromatin binding and activities of the deubiquitinases. Furthermore, we find an uncoupling of the H2Bub1-mediated coregulation of both H3K4 and -K79 methylation, as these H2B C-terminal helix residues are part of a distinct surface that affects only Set1-COMPASS (complex proteins associated with Set1)-mediated H3K4 methylation without affecting the functions of Dot1. Importantly, we also find that these residues interact with Spp1 and control the chromatin association, integrity, and overall stability of Set1-COMPASS independent of H2Bub1. Therefore, we have uncovered a novel role for the H2B C-terminal helix in the trans-histone cross talk as a binding surface for Set1-COMPASS. We provide further insight into the trans-histone cross talk and propose that H2Bub1 stabilizes the nucleosome by preventing H2A-H2B eviction and, thereby, retains the "docking site" for Set1-COMPASS on chromatin to maintain its stable chromatin association, complex stability, and processive methylation.
Figures
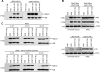


References
-
- Amati, B. B., and S. M. Gasser. 1988. Chromosomal ARS and CEN elements bind specifically to the yeast nuclear scaffold. Cell 54:967-978. - PubMed
-
- Berger, S. L. 2007. The complex language of chromatin regulation during transcription. Nature 447:407-412. - PubMed
-
- Briggs, S. D., T. Xiao, Z. W. Sun, J. A. Caldwell, J. Shabanowitz, D. F. Hunt, C. D. Allis, and B. D. Strahl. 2002. Gene silencing: trans-histone regulatory pathway in chromatin. Nature 418:498. - PubMed
-
- Dehé, P. M., M. Pamblanco, P. Luciano, R. Lebrun, D. Moinier, R. Sendra, A. Verreault, V. Tordera, and V. Géli. 2005. Histone H3 lysine 4 mono-methylation does not require ubiquitination of histone H2B. J. Mol. Biol. 353:477-484. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
