TNF suppresses acute intestinal inflammation by inducing local glucocorticoid synthesis
- PMID: 20439544
- PMCID: PMC2867273
- DOI: 10.1084/jem.20090849
TNF suppresses acute intestinal inflammation by inducing local glucocorticoid synthesis
Abstract
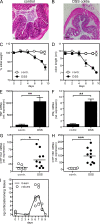
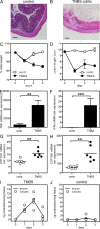
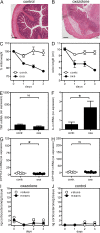
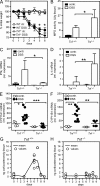
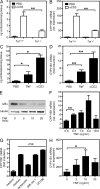
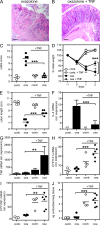
Although tumor necrosis factor (alpha) (TNF) exerts proinflammatory activities in a variety of diseases, including inflammatory bowel disease, there is increasing evidence for antiinflammatory actions of TNF. In contrast, glucocorticoids (GCs) are steroid hormones that suppress inflammation, at least in part by regulating the expression and action of TNF. We report that TNF induces extraadrenal production of immunoregulatory GCs in the intestinal mucosa during acute intestinal inflammation. The absence of TNF results in a lack of colonic GC synthesis and exacerbation of dextran sodium sulfate-induced colitis. TNF seems to promote local steroidogenesis by directly inducing steroidogenic enzymes in intestinal epithelial cells. Therapeutic administration of TNF induces GC synthesis in oxazolone-induced colitis and ameliorates intestinal inflammation, whereas inhibition of intestinal GC synthesis abrogates the therapeutic effect of TNF. These data show that TNF suppresses the pathogenesis of acute intestinal inflammation by promoting local steroidogenesis.
Figures

References
-
- Botrugno O.A., Fayard E., Annicotte J.S., Haby C., Brennan T., Wendling O., Tanaka T., Kodama T., Thomas W., Auwerx J., et al. 2004. Synergy between LRH-1 and beta-catenin induces G1 cyclin-mediated cell proliferation. Mol. Cell. 15:499–509 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

