Gag mutations can impact virological response to dual-boosted protease inhibitor combinations in antiretroviral-naïve HIV-infected patients
- PMID: 20439606
- PMCID: PMC2897283
- DOI: 10.1128/AAC.00194-10
Gag mutations can impact virological response to dual-boosted protease inhibitor combinations in antiretroviral-naïve HIV-infected patients
Abstract
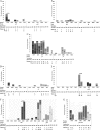
ANRS 127 was a randomized pilot trial involving naïve patients receiving two dual-boosted protease inhibitor (PI) combinations. Virological response, defined as a plasma HIV RNA level of <50 copies/ml at week 16, occurred in only 41% patients. Low baseline plasma HIV RNA level was the only significant predictor of virological response. The purpose of this study was to investigate the impact on virological response of pretherapy mutations in cleavage sites of gag, gag-pol, and the gag-pol frameshift region. The whole gag gene and protease-coding region were amplified and sequenced at baseline and at week 16 for 48 patients still on the allocated regimen at week 16. No major PI resistance-associated mutations were detected either at baseline or in the 26 patients who did not achieve virological response at week 16. Baseline cleavage site substitutions in the product of the gag open reading frame at positions 128 (p17/p24) (P = 0.04) and 449 (p1/p6(gag)) (P = 0.01) were significantly more frequent in those patients not achieving virological response. Conversely, baseline cleavage site mutation at position 437 (TFP/p6(pol)) was associated with virological response (P = 0.04). In multivariate analysis adjusted for baseline viral load, these 3 substitutions remained independently associated with virological response. We demonstrated here, in vivo, an impact of baseline polymorphic gag mutations on virological response in naïve patients receiving a combination of two protease inhibitors. However, it was not possible to link the substitutions selected under PI selective pressure with virological failure.
Figures

References
-
- Bally, F., R. Martinez, S. Peters, P. Sudre, and A. Telenti. 2000. Polymorphism of HIV type 1 gag p7/p1 and p1/p6 cleavage sites: clinical significance and implications for resistance to protease inhibitors. AIDS Res. Hum. Retroviruses 16:1209-1213. - PubMed
-
- Callebaut, C. S., K. Stray, L. Tsai, L. Xu, W. Lee, and T. Cihlar. 2007. In vitro HIV-1 resistance selection to GS-8374, a novel phosphonate protease inhibitor: comparison with lopinavir, atazanavir and darunavir. XVIth Int. HIV Drug Resist. Workshop, abstr. 16.
-
- Carrillo, A., K. D. Stewart, H. L. Sham, D. W. Norbeck, W. E. Kohlbrenner, J. M. Leonard, D. J. Kempf, and A. Molla. 1998. In vitro selection and characterization of human immunodeficiency virus type 1 variants with increased resistance to ABT-378, a novel protease inhibitor. J. Virol. 72:7532-7541. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

