A phase 2 study of eliglustat tartrate (Genz-112638), an oral substrate reduction therapy for Gaucher disease type 1
- PMID: 20439622
- PMCID: PMC2924227
- DOI: 10.1182/blood-2010-03-273151
A phase 2 study of eliglustat tartrate (Genz-112638), an oral substrate reduction therapy for Gaucher disease type 1
Abstract
Eliglustat tartrate (Genz-112638), a specific inhibitor of glucosylceramide synthase, is under development as an oral substrate reduction therapy for Gaucher disease type 1 (GD1). A multinational, open-label, single-arm phase 2 study of 26 GD1 patients (16 female, 10 male; mean age, 34 years) evaluated the efficacy, safety, and pharmacokinetics of eliglustat tartrate administered twice daily by mouth at 50- or 100-mg doses based on plasma drug concentrations. Entry criteria required splenomegaly with thrombocytopenia and/or anemia. The composite primary efficacy end point required improvement after 52 weeks in at least 2 of these 3 disease manifestations and was met by 77% (95% confidence interval [CI] = 58%-89%) of all patients and 91% (95% CI = 72%-98%) of the 22 patients completing 52 weeks. Statistically significant improvements occurred in mean hemoglobin level (1.62 g/dL; 95% CI =1.05-2.18 g/dL), platelet count (40.3%; 95% CI = 23.7-57.0 g/dL), spleen volume (-38.5%; 95% CI = -43.5%--33.5%), liver volume (-17.0%; 95% CI = -21.6%-12.3%), and lumbar spine bone mineral density (0.31 Z-score; 95% CI = 0.09-0.53). Elevated biomarkers (chitotriosidase; chemokine CCL18; angiotensin-converting enzyme; tartrate-resistant acid phosphatase) decreased by 35% to 50%. Plasma glucosylceramide and ganglioside GM3 normalized. Eliglustat tartrate was well tolerated: 7 mild, transient adverse events in 6 patients were considered treatment-related. Individual pharmacokinetics varied; mean time to maximal observed concentration was 2.3 hours and mean half-life was 6.8 hours. Eliglustat tartrate appears to be a promising oral treatment for GD1.
Trial registration: ClinicalTrials.gov NCT00358150.
Figures
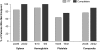
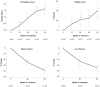
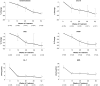
Comment in
-
The substrate is down: is the IV out?Blood. 2010 Aug 12;116(6):860-1. doi: 10.1182/blood-2010-05-285163. Blood. 2010. PMID: 20705763 No abstract available.
References
-
- Beutler E, Grabowski G. Gaucher disease. In: Scriver CR, Beaudet A, Sly W, et al., editors. The Metabolic and Molecular Bases of Inherited Disease. New York, NY: McGraw-Hill; 2001. pp. 3635–3668.
-
- Grabowski GA, Barton NW, Pastores G, et al. Enzyme therapy in type 1 Gaucher disease: comparative efficacy of mannose-terminated glucocerebrosidase from natural and recombinant sources. Ann Intern Med. 1995;122(1):33–39. - PubMed
-
- Pastores GM, Weinreb NJ, Aerts H, et al. Therapeutic goals in the treatment of Gaucher disease. Semin Hematol. 2004;41(4) suppl 5:4–14. - PubMed
-
- Weinreb NJ, Charrow J, Andersson HC, et al. Effectiveness of enzyme replacement therapy in 1028 patients with type 1 Gaucher disease after 2 to 5 years of treatment: a report from the Gaucher Registry. Am J Med. 2002;113(2):112–119. - PubMed
-
- Vunnam RR, Radin NS. Analogs of ceramide that inhibit glucocerebroside synthetase in mouse brain. Chem Phys Lipids. 1980;26(3):265–278. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

