Derivation and characterization of an extra-axial chordoma cell line (EACH-1) from a scapular tumor
- PMID: 20439670
- PMCID: PMC7000130
- DOI: 10.2106/JBJS.I.00594
Derivation and characterization of an extra-axial chordoma cell line (EACH-1) from a scapular tumor
Abstract
Background: Extra-axial chordomas are rare low-grade malignant tumors thought to arise from notochordal remnants in the extra-axial skeleton. Few studies have been done on this neoplasm because of its rarity. In addition, there is a lack of a good in vitro model on which to perform more characterization.
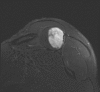
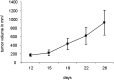
Methods: We describe a twenty-eight-year-old man with a mass in the right scapula. Cytomorphology and immunohistochemistry, including brachyury staining, were used to formulate the final diagnosis. A fragment of the tumor was placed in culture, and cells obtained were injected subcutaneously in an immunocompromised mouse. From the tumor developed in mice, a cell line has been derived and characterized by fluorescence-activated cell-sorting analysis, karyotyping, clonogenicity, and cell and tumor growth curves.
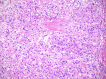
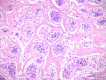
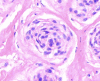
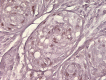
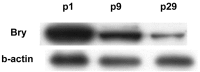
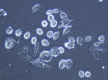
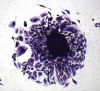
Results: Cytomorphology on the tumor showed nests of round cells with vacuoles and also physaliferous-like cells with uniform nuclei. Immunochemistry revealed a tumor positive for vimentin, moderately positive for S-100 and cytokeratin AE1/AE3, weakly positive for epithelial membrane antigen, and negative for p63 and cytokeratin (CK)-7. Further analysis revealed the tumor was diffusely and strongly positive for brachyury. The cell line derived from the tumor showed rapid doubling-time, a strong expression of mesenchymal cell surface markers, a karyotype of diploid or hypotetraploid clones with numerous chromosomal aberrations, and the ability to form colonies without attachment and to form tumors in immunocompromised mice.
Conclusions: The diagnosis of the extra-axial chordoma is difficult but can be resolved by the detection of a strong brachyury expression. In addition, the derivation of a human extra-axial chordoma cell line could be a useful tool for the basic research of this rare neoplasm.
Figures






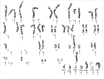
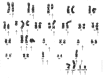

References
-
- Bjornsson J Wold LE Ebersold MJ Laws ER. Chordoma of the mobile spine. A clinicopathologic analysis of 40 patients. Cancer. 1993;71:735-40. - PubMed
-
- McMaster ML Goldstein AM Bromley CM Ishibe N Parry DM. Chordoma: incidence and survival patterns in the United States, 1973-1995. Cancer Causes Control. 2001;12:1-11. - PubMed
-
- Heffelfinger MJ Dahlin DC MacCarty CS Beabout JW. Chordomas and cartilaginous tumors at the skull base. Cancer. 1973;32:410-20. - PubMed
-
- Folpe AL Agoff SN Willis J Weiss SW. Parachordoma is immunohistochemically and cytogenetically distinct from axial chordoma and extraskeletal myxoid chondrosarcoma. Am J Surg Pathol. 1999;23:1059-67. - PubMed
-
- Kilpatrick SE Hitchcock MG Kraus MD Calonje E Fletcher CD. Mixed tumors and myoepitheliomas of soft tissue: a clinicopathologic study of 19 cases with a unifying concept. Am J Surg Pathol. 1997;21:13-22. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

