Gluconeogenic carbon flow of tricarboxylic acid cycle intermediates is critical for Mycobacterium tuberculosis to establish and maintain infection
- PMID: 20439709
- PMCID: PMC2906907
- DOI: 10.1073/pnas.1000715107
Gluconeogenic carbon flow of tricarboxylic acid cycle intermediates is critical for Mycobacterium tuberculosis to establish and maintain infection
Abstract
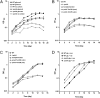
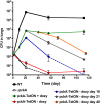
Metabolic adaptation to the host niche is a defining feature of the pathogenicity of Mycobacterium tuberculosis (Mtb). In vitro, Mtb is able to grow on a variety of carbon sources, but mounting evidence has implicated fatty acids as the major source of carbon and energy for Mtb during infection. When bacterial metabolism is primarily fueled by fatty acids, biosynthesis of sugars from intermediates of the tricarboxylic acid cycle is essential for growth. The role of gluconeogenesis in the pathogenesis of Mtb however remains unaddressed. Phosphoenolpyruvate carboxykinase (PEPCK) catalyzes the first committed step of gluconeogenesis. We applied genetic analyses and (13)C carbon tracing to confirm that PEPCK is essential for growth of Mtb on fatty acids and catalyzes carbon flow from tricarboxylic acid cycle-derived metabolites to gluconeogenic intermediates. We further show that PEPCK is required for growth of Mtb in isolated bone marrow-derived murine macrophages and in mice. Importantly, Mtb lacking PEPCK not only failed to replicate in mouse lungs but also failed to survive, and PEPCK depletion during the chronic phase of infection resulted in mycobacterial clearance. Mtb thus relies on gluconeogenesis throughout the infection. PEPCK depletion also attenuated Mtb in IFNgamma-deficient mice, suggesting that this enzyme represents an attractive target for chemotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

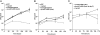

References
-
- Harries AD, Dye C. Tuberculosis. Ann Trop Med Parasitol. 2006;100:415–431. - PubMed
-
- Boshoff HI, Barry CE., 3rd Tuberculosis—metabolism and respiration in the absence of growth. Nat Rev Microbiol. 2005;3:70–80. - PubMed
-
- Muñoz-Elías EJ, McKinney JD. Carbon metabolism of intracellular bacteria. Cell Microbiol. 2006;8:10–22. - PubMed
-
- Sauer U, Eikmanns BJ. The PEP-pyruvate-oxaloacetate node as the switch point for carbon flux distribution in bacteria. FEMS Microbiol Rev. 2005;29:765–794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

