A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides
- PMID: 20439716
- PMCID: PMC2889104
- DOI: 10.1073/pnas.1000675107
A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides
Abstract
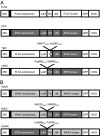
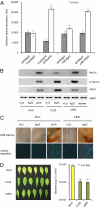
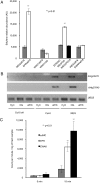
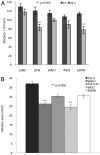
Oligogalacturonides (OGs) released from the plant cell wall are active both as damage-associated molecular patterns (DAMPs) for the activation of the plant immune response and regulators of plant growth and development. Members of the Wall-Associated Kinase (WAK) family are candidate receptors of OGs, due to their ability to bind in vitro these oligosaccharides. Because lethality and redundancy have hampered the study of WAKs by reverse genetics, we have adopted a chimeric receptor approach to elucidate the role of Arabidopsis WAK1. In a test-of-concept study, we first defined the appropriate chimera design and demonstrated that the Arabidopsis pattern recognition receptor (PRR) EFR is amenable to the construction of functional and resistance-conferring chimeric receptors carrying the ectodomain of another Arabidopsis PRR, FLS2. After, we analyzed chimeras derived from EFR and WAK1. Our results show that, upon stimulation with OGs, the WAK1 ectodomain is capable of activating the EFR kinase domain. On the other hand, upon stimulation with the cognate ligand elf18, the EFR ectodomain activates the WAK1 kinase, triggering defense responses that mirror those normally activated by OGs and are effective against fungal and bacterial pathogens. Finally, we show that transgenic plants overexpressing WAK1 are more resistant to Botrytis cinerea.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–461. - PubMed
-
- Scheibner KA, et al. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol. 2006;177:1272–1281. - PubMed
-
- Willats WG, McCartney L, Mackie W, Knox JP. Pectin: Cell biology and prospects for functional analysis. Plant Mol Biol. 2001;47:9–27. - PubMed
-
- Ridley BL, O'Neill MA, Mohnen D. Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry. 2001;57:929–967. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

