Neural basis of global resting-state fMRI activity
- PMID: 20439733
- PMCID: PMC2890438
- DOI: 10.1073/pnas.0913110107
Neural basis of global resting-state fMRI activity
Abstract
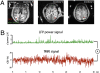
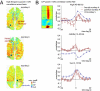
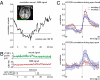
Functional MRI (fMRI) has uncovered widespread hemodynamic fluctuations in the brain during rest. Recent electroencephalographic work in humans and microelectrode recordings in anesthetized monkeys have shown this activity to be correlated with slow changes in neural activity. Here we report that the spontaneous fluctuations in the local field potential (LFP) measured from a single cortical site in monkeys at rest exhibit widespread, positive correlations with fMRI signals over nearly the entire cerebral cortex. This correlation was especially consistent in a band of upper gamma-range frequencies (40-80 Hz), for which the hemodynamic signal lagged the neural signal by 6-8 s. A strong, positive correlation was also observed in a band of lower frequencies (2-15 Hz), albeit with a lag closer to zero. The global pattern of correlation with spontaneous fMRI fluctuations was similar whether the LFP signal was measured in occipital, parietal, or frontal electrodes. This coupling was, however, dependent on the monkey's behavioral state, being stronger and anticipatory when the animals' eyes were closed. These results indicate that the often discarded global component of fMRI fluctuations measured during the resting state is tightly coupled with underlying neural activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Neuronal correlate of BOLD signal fluctuations at rest: err on the side of the baseline.Proc Natl Acad Sci U S A. 2010 Jun 15;107(24):10773-4. doi: 10.1073/pnas.1005135107. Epub 2010 Jun 8. Proc Natl Acad Sci U S A. 2010. PMID: 20534504 Free PMC article. No abstract available.
References
-
- Arieli A, Shoham D, Hildesheim R, Grinvald A. Coherent spatiotemporal patterns of ongoing activity revealed by real-time optical imaging coupled with single-unit recording in the cat visual cortex. J Neurophysiol. 1995;73:2072–2093. - PubMed
-
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: Explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. - PubMed
-
- Leopold DA, Logothetis NK. Spatial patterns of spontaneous local field activity in the monkey visual cortex. Rev Neurosci. 2003;14:195–205. - PubMed
-
- Leopold DA, Murayama Y, Logothetis NK. Very slow activity fluctuations in monkey visual cortex: Implications for functional brain imaging. Cereb Cortex. 2003;13:422–433. - PubMed
-
- Snodderly DM, Gur M. Organization of striate cortex of alert, trained monkeys (Macaca fascicularis): Ongoing activity, stimulus selectivity, and widths of receptive field activating regions. J Neurophysiol. 1995;74:2100–2125. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

