Identification of select glucocorticoids as Smoothened agonists: potential utility for regenerative medicine
- PMID: 20439738
- PMCID: PMC2889058
- DOI: 10.1073/pnas.0910712107
Identification of select glucocorticoids as Smoothened agonists: potential utility for regenerative medicine
Abstract
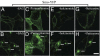
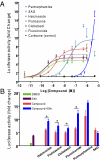
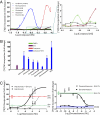
Regenerative medicine holds the promise of replacing damaged tissues largely by stem cell activation. Hedgehog signaling through the plasma membrane receptor Smoothened (Smo) is an important process for regulating stem cell proliferation. The development of Hedgehog-related therapies has been impeded by a lack of US Food and Drug Administration (FDA)-approved Smo agonists. Using a high-content screen with cells expressing Smo receptors and a beta-arrestin2-GFP reporter, we identified four FDA-approved drugs, halcinonide, fluticasone, clobetasol, and fluocinonide, as Smo agonists that activate Hedgehog signaling. These drugs demonstrated an ability to bind Smo, promote Smo internalization, activate Gli, and stimulate the proliferation of primary neuronal precursor cells alone and synergistically in the presence of Sonic Hedgehog protein. Halcinonide, fluticasone, clobetasol, and fluocinonide provide an unprecedented opportunity to develop unique clinical strategies to treat Hedgehog-dependent illnesses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: Paradigms and principles. Genes Dev. 2001;15:3059–3087. - PubMed
-
- Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6:21–27. - PubMed
-
- Dahmane N, Ruiz i Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126:3089–3100. - PubMed
-
- Machold R, et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–950. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

