Electrophilic tuning of the chemoprotective natural product sulforaphane
- PMID: 20439747
- PMCID: PMC2906893
- DOI: 10.1073/pnas.1004104107
Electrophilic tuning of the chemoprotective natural product sulforaphane
Abstract
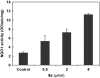
Sulforaphane [1-isothiocyanato-4-(methylsulfinyl)butane], a naturally occurring isothiocyanate derived from cruciferous vegetables, is a highly potent inducer of phase 2 cytoprotective enzymes and can protect against electrophiles including carcinogens, oxidative stress, and inflammation. The mechanism of action of sulforaphane is believed to involve modifications of critical cysteine residues of Keap1, which lead to stabilization of Nrf2 to activate the antioxidant response element of phase 2 enzymes. However, the dithiocarbamate functional group formed by a reversible reaction between isothiocyanate of sulforaphane and sulfhydryl nucleophiles of Keap1 is kinetically labile, and such modification in intact cells has not yet been demonstrated. Here we designed sulforaphane analogs with replacement of the reactive isothiocyanate by the more gentle electrophilic sulfoxythiocarbamate group that also selectively targets cysteine residues in proteins but forms stable thiocarbamate adducts. Twenty-four sulfoxythiocarbamate analogs were synthesized that retain the structural features important for high potency in sulforaphane analogs: the sulfoxide or keto group and its appropriate distance to electrophilic functional group. Evaluation in various cell lines including hepatoma cells, retinal pigment epithelial cells, and keratinocytes as well as in mouse skin shows that these analogs maintain high potency and efficacy for phase 2 enzyme induction as well as the inhibitory effect on lipopolysaccharide-induced nitric oxide formation like sulforaphane. We further show in living cells that a sulfoxythiocarbamate analog can label Keap1 on several key cysteine residues as well as other cellular proteins offering new insights into the mechanism of chemoprotection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

