Recombinant yeast screen for new inhibitors of human acetyl-CoA carboxylase 2 identifies potential drugs to treat obesity
- PMID: 20439761
- PMCID: PMC2889071
- DOI: 10.1073/pnas.1003721107
Recombinant yeast screen for new inhibitors of human acetyl-CoA carboxylase 2 identifies potential drugs to treat obesity
Abstract
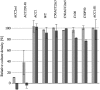
Acetyl-CoA carboxylase (ACC) is a key enzyme of fatty acid metabolism with multiple isozymes often expressed in different eukaryotic cellular compartments. ACC-made malonyl-CoA serves as a precursor for fatty acids; it also regulates fatty acid oxidation and feeding behavior in animals. ACC provides an important target for new drugs to treat human diseases. We have developed an inexpensive nonradioactive high-throughput screening system to identify new ACC inhibitors. The screen uses yeast gene-replacement strains depending for growth on cloned human ACC1 and ACC2. In "proof of concept" experiments, growth of such strains was inhibited by compounds known to target human ACCs. The screen is sensitive and robust. Medium-size chemical libraries yielded new specific inhibitors of human ACC2. The target of the best of these inhibitors was confirmed with in vitro enzymatic assays. This compound is a new drug chemotype inhibiting human ACC2 with 2.8 muM IC(50) and having no effect on human ACC1 at 100 muM.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


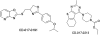
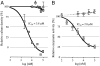
References
-
- Hofer U, Muehlebach M, Hole S, Zoschke A. Pinoxaden-for broad spectrum grass weed management in cereal crops. J Plant Dis Protect. 2006;20:889–995.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

