Single residue within the antigen translocation complex TAP controls the epitope repertoire by stabilizing a receptive conformation
- PMID: 20439763
- PMCID: PMC2889111
- DOI: 10.1073/pnas.1001308107
Single residue within the antigen translocation complex TAP controls the epitope repertoire by stabilizing a receptive conformation
Abstract
The recognition of virus infected or malignantly transformed cells by cytotoxic T lymphocytes critically depends on the transporter associated with antigen processing (TAP), which delivers proteasomal degradation products into the endoplasmic reticulum lumen for subsequent loading of major histocompatibility complex class I molecules. Here we have identified a single cysteinyl residue in the TAP complex that modulates peptide binding and translocation, thereby restricting the epitope repertoire. Cysteine 213 in human TAP2 was found to be part of a newly uncovered substrate-binding site crucial for peptide recognition. This residue contacts the peptide in the binding pocket in an orientated manner. The translocation complex can be reversibly inactivated by thiol modification of this cysteinyl residue. As part of an unexpected mechanism, this residue is crucial in complementing the binding pocket for a given subset of epitopes as well as in maintaining a substrate-receptive conformation of the translocation complex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
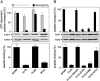
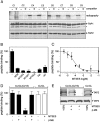
 ). Cross-linking was induced by adding 1 mM of copper phenanthroline in the presence or absence of the competitor RRYQKSTEL (0.25 mM). After metal affinity purification of the TAP complex, cross-linked products were analyzed by nonreducing SDS-PAGE (10%) and autoradiography. The TAP expression was confirmed by SDS-PAGE and immunoblotting against TAP1 and TAP2 (mAb148.3 and mAb435.3, respectively). The asterisk indicates a TAP1 degradation product. (B) Modification of C213 blocks peptide binding of TAP. CL/CL(C213) containing microsomes (20 μg of total protein) were incubated with various thiol-specific reagents [0.5 mM NEM; 10 mM MTSES; 1 mM [2-{trimethylammonium)ethyl]methanethiosulfonate; 2.5 mM (2-aminoethyl)methanethiosulfonate; 0.25 mM fluorescein-5-maleimide (F-Mal); 0.25 mM 5-iodoacetamidofluorescein (5-IAF)] for 15 min on ice. Subsequently, peptide-binding assays were performed using radiolabeled RR(125I)YQKSTEL (1 μM) as a reporter. (C) Inhibition of peptide binding by MTSES. To determine the half-maximum inhibition value (IC50) of MTSES, CL/CL(C213) containing microsomes were incubated with an increasing concentration of MTSES followed by peptide-binding studies under conditions as described in Fig. 2A. The IC50 was determined to be 52 ± 7 μM (Eq. S2). (D) Reversible TAP inhibition by thiol-specific modification. CL/CL(C213) containing microsomes (20 μg of total protein) were incubated with 10 mM of MTSES for 15 min on ice and subsequently incubated with or without β-ME (100 mM) for 30 min on ice. Peptide binding was assayed with 1 μM of radiolabeled RR(125I)YQKSTEL. (E) Efficiency of MTS labeling was determined by means of accessibility for alkylation by 5-IAF. After MTS labeling, samples were incubated with 5-IAF (250 μM) for 15 min on ice and the relative amounts of 5-IAF-modified TAP were determined by in-gel fluorescence. To determine the maximal labeling capacity by 5-IAF (shown as 100%), TAP was denatured by SDS (2%) for 20 min at room temperature and then labeled with 250 μM of 5-IAF for 3 min before SDS-PAGE (10%) and immunoblotting. Asterisks indicate nonspecific labeling.
). Cross-linking was induced by adding 1 mM of copper phenanthroline in the presence or absence of the competitor RRYQKSTEL (0.25 mM). After metal affinity purification of the TAP complex, cross-linked products were analyzed by nonreducing SDS-PAGE (10%) and autoradiography. The TAP expression was confirmed by SDS-PAGE and immunoblotting against TAP1 and TAP2 (mAb148.3 and mAb435.3, respectively). The asterisk indicates a TAP1 degradation product. (B) Modification of C213 blocks peptide binding of TAP. CL/CL(C213) containing microsomes (20 μg of total protein) were incubated with various thiol-specific reagents [0.5 mM NEM; 10 mM MTSES; 1 mM [2-{trimethylammonium)ethyl]methanethiosulfonate; 2.5 mM (2-aminoethyl)methanethiosulfonate; 0.25 mM fluorescein-5-maleimide (F-Mal); 0.25 mM 5-iodoacetamidofluorescein (5-IAF)] for 15 min on ice. Subsequently, peptide-binding assays were performed using radiolabeled RR(125I)YQKSTEL (1 μM) as a reporter. (C) Inhibition of peptide binding by MTSES. To determine the half-maximum inhibition value (IC50) of MTSES, CL/CL(C213) containing microsomes were incubated with an increasing concentration of MTSES followed by peptide-binding studies under conditions as described in Fig. 2A. The IC50 was determined to be 52 ± 7 μM (Eq. S2). (D) Reversible TAP inhibition by thiol-specific modification. CL/CL(C213) containing microsomes (20 μg of total protein) were incubated with 10 mM of MTSES for 15 min on ice and subsequently incubated with or without β-ME (100 mM) for 30 min on ice. Peptide binding was assayed with 1 μM of radiolabeled RR(125I)YQKSTEL. (E) Efficiency of MTS labeling was determined by means of accessibility for alkylation by 5-IAF. After MTS labeling, samples were incubated with 5-IAF (250 μM) for 15 min on ice and the relative amounts of 5-IAF-modified TAP were determined by in-gel fluorescence. To determine the maximal labeling capacity by 5-IAF (shown as 100%), TAP was denatured by SDS (2%) for 20 min at room temperature and then labeled with 250 μM of 5-IAF for 3 min before SDS-PAGE (10%) and immunoblotting. Asterisks indicate nonspecific labeling.
Similar articles
-
Nucleotide binding by TAP mediates association with peptide and release of assembled MHC class I molecules.Curr Biol. 1999 Sep 23;9(18):999-1008. doi: 10.1016/s0960-9822(99)80448-5. Curr Biol. 1999. PMID: 10508608
-
Characteristics of peptide and major histocompatibility complex class I/beta 2-microglobulin binding to the transporters associated with antigen processing (TAP1 and TAP2).Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12716-20. doi: 10.1073/pnas.91.26.12716. Proc Natl Acad Sci U S A. 1994. PMID: 7809108 Free PMC article.
-
Processing of a multiple membrane spanning Epstein-Barr virus protein for CD8(+) T cell recognition reveals a proteasome-dependent, transporter associated with antigen processing-independent pathway.J Exp Med. 2001 Oct 15;194(8):1053-68. doi: 10.1084/jem.194.8.1053. J Exp Med. 2001. PMID: 11602636 Free PMC article.
-
The transporter associated with antigen processing: a key player in adaptive immunity.Biol Chem. 2015 Sep;396(9-10):1059-72. doi: 10.1515/hsz-2014-0320. Biol Chem. 2015. PMID: 25781678 Review.
-
The ABCs of immunology: structure and function of TAP, the transporter associated with antigen processing.Physiology (Bethesda). 2004 Aug;19:216-24. doi: 10.1152/physiol.00002.2004. Physiology (Bethesda). 2004. PMID: 15304636 Review.
Cited by
-
Structure and Dynamics of Antigenic Peptides in Complex with TAP.Front Immunol. 2017 Jan 30;8:10. doi: 10.3389/fimmu.2017.00010. eCollection 2017. Front Immunol. 2017. PMID: 28194151 Free PMC article. Review.
-
Mechanics and pharmacology of substrate selection and transport by eukaryotic ABC exporters.Nat Struct Mol Biol. 2019 Sep;26(9):792-801. doi: 10.1038/s41594-019-0280-4. Epub 2019 Aug 26. Nat Struct Mol Biol. 2019. PMID: 31451804 Free PMC article. Review.
-
A dual inhibition mechanism of herpesviral ICP47 arresting a conformationally thermostable TAP complex.Sci Rep. 2016 Nov 15;6:36907. doi: 10.1038/srep36907. Sci Rep. 2016. PMID: 27845362 Free PMC article.
-
Characterization and allelic variation of the transporters associated with antigen processing (TAP) genes in the domestic dog (Canis lupus familiaris).Dev Comp Immunol. 2013 Dec;41(4):578-86. doi: 10.1016/j.dci.2013.07.011. Epub 2013 Jul 25. Dev Comp Immunol. 2013. PMID: 23892057 Free PMC article.
-
Crystal structure and mechanistic basis of a functional homolog of the antigen transporter TAP.Proc Natl Acad Sci U S A. 2017 Jan 24;114(4):E438-E447. doi: 10.1073/pnas.1620009114. Epub 2017 Jan 9. Proc Natl Acad Sci U S A. 2017. PMID: 28069938 Free PMC article.
References
-
- Lehner PJ, Cresswell P. Recent developments in MHC-class-I-mediated antigen presentation. Curr Opin Immunol. 2004;16:82–89. - PubMed
-
- Rock KL, et al. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. - PubMed
-
- Yewdell JW, Haeryfar SM. Understanding presentation of viral antigens to CD8+ T cells in vivo: The key to rational vaccine design. Annu Rev Immunol. 2005;23:651–682. - PubMed
-
- Abele R, Tampé R. Peptide trafficking and translocation across membranes in cellular signaling and self-defense strategies. Curr Opin Cell Biol. 2009;21:508–515. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

