Single residue within the antigen translocation complex TAP controls the epitope repertoire by stabilizing a receptive conformation
- PMID: 20439763
- PMCID: PMC2889111
- DOI: 10.1073/pnas.1001308107
Single residue within the antigen translocation complex TAP controls the epitope repertoire by stabilizing a receptive conformation
Abstract
The recognition of virus infected or malignantly transformed cells by cytotoxic T lymphocytes critically depends on the transporter associated with antigen processing (TAP), which delivers proteasomal degradation products into the endoplasmic reticulum lumen for subsequent loading of major histocompatibility complex class I molecules. Here we have identified a single cysteinyl residue in the TAP complex that modulates peptide binding and translocation, thereby restricting the epitope repertoire. Cysteine 213 in human TAP2 was found to be part of a newly uncovered substrate-binding site crucial for peptide recognition. This residue contacts the peptide in the binding pocket in an orientated manner. The translocation complex can be reversibly inactivated by thiol modification of this cysteinyl residue. As part of an unexpected mechanism, this residue is crucial in complementing the binding pocket for a given subset of epitopes as well as in maintaining a substrate-receptive conformation of the translocation complex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
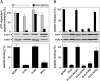
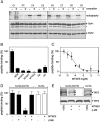
 ). Cross-linking was induced by adding 1 mM of copper phenanthroline in the presence or absence of the competitor RRYQKSTEL (0.25 mM). After metal affinity purification of the TAP complex, cross-linked products were analyzed by nonreducing SDS-PAGE (10%) and autoradiography. The TAP expression was confirmed by SDS-PAGE and immunoblotting against TAP1 and TAP2 (mAb148.3 and mAb435.3, respectively). The asterisk indicates a TAP1 degradation product. (B) Modification of C213 blocks peptide binding of TAP. CL/CL(C213) containing microsomes (20 μg of total protein) were incubated with various thiol-specific reagents [0.5 mM NEM; 10 mM MTSES; 1 mM [2-{trimethylammonium)ethyl]methanethiosulfonate; 2.5 mM (2-aminoethyl)methanethiosulfonate; 0.25 mM fluorescein-5-maleimide (F-Mal); 0.25 mM 5-iodoacetamidofluorescein (5-IAF)] for 15 min on ice. Subsequently, peptide-binding assays were performed using radiolabeled RR(125I)YQKSTEL (1 μM) as a reporter. (C) Inhibition of peptide binding by MTSES. To determine the half-maximum inhibition value (IC50) of MTSES, CL/CL(C213) containing microsomes were incubated with an increasing concentration of MTSES followed by peptide-binding studies under conditions as described in Fig. 2A. The IC50 was determined to be 52 ± 7 μM (Eq. S2). (D) Reversible TAP inhibition by thiol-specific modification. CL/CL(C213) containing microsomes (20 μg of total protein) were incubated with 10 mM of MTSES for 15 min on ice and subsequently incubated with or without β-ME (100 mM) for 30 min on ice. Peptide binding was assayed with 1 μM of radiolabeled RR(125I)YQKSTEL. (E) Efficiency of MTS labeling was determined by means of accessibility for alkylation by 5-IAF. After MTS labeling, samples were incubated with 5-IAF (250 μM) for 15 min on ice and the relative amounts of 5-IAF-modified TAP were determined by in-gel fluorescence. To determine the maximal labeling capacity by 5-IAF (shown as 100%), TAP was denatured by SDS (2%) for 20 min at room temperature and then labeled with 250 μM of 5-IAF for 3 min before SDS-PAGE (10%) and immunoblotting. Asterisks indicate nonspecific labeling.
). Cross-linking was induced by adding 1 mM of copper phenanthroline in the presence or absence of the competitor RRYQKSTEL (0.25 mM). After metal affinity purification of the TAP complex, cross-linked products were analyzed by nonreducing SDS-PAGE (10%) and autoradiography. The TAP expression was confirmed by SDS-PAGE and immunoblotting against TAP1 and TAP2 (mAb148.3 and mAb435.3, respectively). The asterisk indicates a TAP1 degradation product. (B) Modification of C213 blocks peptide binding of TAP. CL/CL(C213) containing microsomes (20 μg of total protein) were incubated with various thiol-specific reagents [0.5 mM NEM; 10 mM MTSES; 1 mM [2-{trimethylammonium)ethyl]methanethiosulfonate; 2.5 mM (2-aminoethyl)methanethiosulfonate; 0.25 mM fluorescein-5-maleimide (F-Mal); 0.25 mM 5-iodoacetamidofluorescein (5-IAF)] for 15 min on ice. Subsequently, peptide-binding assays were performed using radiolabeled RR(125I)YQKSTEL (1 μM) as a reporter. (C) Inhibition of peptide binding by MTSES. To determine the half-maximum inhibition value (IC50) of MTSES, CL/CL(C213) containing microsomes were incubated with an increasing concentration of MTSES followed by peptide-binding studies under conditions as described in Fig. 2A. The IC50 was determined to be 52 ± 7 μM (Eq. S2). (D) Reversible TAP inhibition by thiol-specific modification. CL/CL(C213) containing microsomes (20 μg of total protein) were incubated with 10 mM of MTSES for 15 min on ice and subsequently incubated with or without β-ME (100 mM) for 30 min on ice. Peptide binding was assayed with 1 μM of radiolabeled RR(125I)YQKSTEL. (E) Efficiency of MTS labeling was determined by means of accessibility for alkylation by 5-IAF. After MTS labeling, samples were incubated with 5-IAF (250 μM) for 15 min on ice and the relative amounts of 5-IAF-modified TAP were determined by in-gel fluorescence. To determine the maximal labeling capacity by 5-IAF (shown as 100%), TAP was denatured by SDS (2%) for 20 min at room temperature and then labeled with 250 μM of 5-IAF for 3 min before SDS-PAGE (10%) and immunoblotting. Asterisks indicate nonspecific labeling.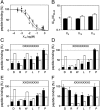
References
-
- Lehner PJ, Cresswell P. Recent developments in MHC-class-I-mediated antigen presentation. Curr Opin Immunol. 2004;16:82–89. - PubMed
-
- Rock KL, et al. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. - PubMed
-
- Yewdell JW, Haeryfar SM. Understanding presentation of viral antigens to CD8+ T cells in vivo: The key to rational vaccine design. Annu Rev Immunol. 2005;23:651–682. - PubMed
-
- Abele R, Tampé R. Peptide trafficking and translocation across membranes in cellular signaling and self-defense strategies. Curr Opin Cell Biol. 2009;21:508–515. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

