Statistical design and analysis of RNA sequencing data
- PMID: 20439781
- PMCID: PMC2881125
- DOI: 10.1534/genetics.110.114983
Statistical design and analysis of RNA sequencing data
Abstract
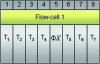
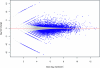
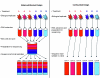
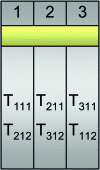
Next-generation sequencing technologies are quickly becoming the preferred approach for characterizing and quantifying entire genomes. Even though data produced from these technologies are proving to be the most informative of any thus far, very little attention has been paid to fundamental design aspects of data collection and analysis, namely sampling, randomization, replication, and blocking. We discuss these concepts in an RNA sequencing framework. Using simulations we demonstrate the benefits of collecting replicated RNA sequencing data according to well known statistical designs that partition the sources of biological and technical variation. Examples of these designs and their corresponding models are presented with the goal of testing differential expression.
Figures



References
-
- Agresti, A., 2002. Categorical Data Analysis, Ed. 2. Wiley, Hoboken, NJ.
-
- Audic, S., and J. Claverie, 1997. The significance of digital gene expression profiles. Genome Res. 7 986–995. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

