Surfactant protein-A as an anti-inflammatory component in the amnion: implications for human pregnancy
- PMID: 20439915
- PMCID: PMC3103775
- DOI: 10.4049/jimmunol.0903867
Surfactant protein-A as an anti-inflammatory component in the amnion: implications for human pregnancy
Abstract
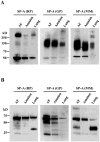
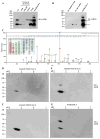
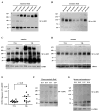
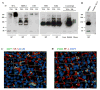
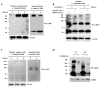
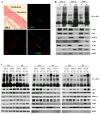
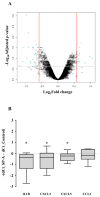
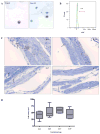
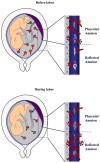
The mechanism of mouse parturition is thought to involve myometrial infiltration by amniotic fluid (AF) macrophages, activated by surfactant protein-A (SP-A). In humans, the concentration of AF SP-A decreases during labor, and no fetal macrophages are found in the myometrium after labor. Therefore, it appears that the mechanisms of labor in mice and humans are different. We investigated a potential role for SP-A in human pregnancy and parturition by examining SP-A expression patterns in AF and amnion. High molecular mass (>250 kDa) oligomeric SP-A was increased in AF with advancing gestation. Interestingly, these oligomers were more abundant in placental amnion before labor at term, while they increased primarily in reflected amnion during labor (p < 0.05). Immunoblotting showed a binding of high molecular mass SP-A in AF to amnion. In C57BL/6 mice, oligomeric SP-A was also readily detected in AF from E15 onwards, but not in amnion. Macrophage density in mice myometrium did not change with advancing gestational age. Microarray analysis of human amnion explants incubated with SP-A revealed a molecular signature of inhibited cytokine-cytokine receptor interaction with downregulation of IL-1beta, CXCL2, and CXCL5 mRNA expression. The findings in this study strongly suggest that SP-A signals amniotic anti-inflammatory response via AF during pregnancy. We propose that an SP-A interaction among AF, placental amnion, and reflected amnion is a unique mechanism for immunoregulation in human pregnancy akin to that established in lung biology. However, AF SP-A and fetal macrophages by themselves do not seem to be exclusive effectors of parturition in humans.
Figures
References
-
- Millar LK, Stollberg J, Debuque L, Bryant-Greenwood G. Fetal membrane distention: determination of the intrauterine surface area and distention of the fetal membranes preterm and at term. Am J Obstet Gynecol. 2000;182:128–134. - PubMed
-
- Mitchell CM, Johnson RF, Giles WB, Zakar T. Prostaglandin H synthase-2 gene regulation in the amnion at labour: histone acetylation and nuclear factor kappa B binding to the promoter in vivo. Mol Hum Reprod. 2008;14:53–59. - PubMed
-
- Romero R, Durum S, Dinarello CA, Oyarzun E, Hobbins JC, Mitchell MD. Interleukin-1 stimulates prostaglandin biosynthesis by human amnion. Prostaglandins. 1989;37:13–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

