Osteopotentia regulates osteoblast maturation, bone formation, and skeletal integrity in mice
- PMID: 20440000
- PMCID: PMC2867309
- DOI: 10.1083/jcb.201003006
Osteopotentia regulates osteoblast maturation, bone formation, and skeletal integrity in mice
Abstract
During skeletal development and regeneration, bone-forming osteoblasts respond to high metabolic demand by active expansion of their rough endoplasmic reticulum (rER) and increased synthesis of type I collagen, the predominant bone matrix protein. However, the molecular mechanisms that orchestrate this response are not well understood. We show that insertional mutagenesis of the previously uncharacterized osteopotentia (Opt) gene disrupts osteoblast function and causes catastrophic defects in postnatal skeletal development. Opt encodes a widely expressed rER-localized integral membrane protein containing a conserved SUN (Sad1/Unc-84 homology) domain. Mice lacking Opt develop acute onset skeletal defects that include impaired bone formation and spontaneous fractures. These defects result in part from a cell-autonomous failure of osteoblast maturation and a posttranscriptional decline in type I collagen synthesis, which is concordant with minimal rER expansion. By identifying Opt as a crucial regulator of bone formation in the mouse, our results uncover a novel rER-mediated control point in osteoblast function and implicate human Opt as a candidate gene for brittle bone disorders.
Figures
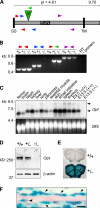

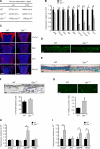


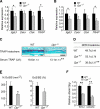
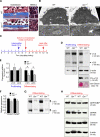
References
-
- Baker S.L. 1956. Fibrogenesis imperfecta ossium; a generalised disease of bone characterised by defective formation of the collagen fibres of the bone matrix. J. Bone Joint Surg. Br. 38-B:378–417 - PubMed
-
- Baker J., Liu J.P., Robertson E.J., Efstratiadis A. 1993. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 75:73–82 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

