Hypoxia-inducible factor-1alpha regulates beta cell function in mouse and human islets
- PMID: 20440072
- PMCID: PMC2877560
- DOI: 10.1172/JCI35846
Hypoxia-inducible factor-1alpha regulates beta cell function in mouse and human islets
Abstract
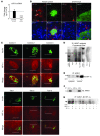
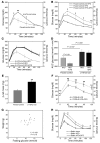
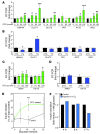
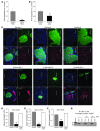
Hypoxia-inducible factor-1alpha (HIF-1alpha) is a transcription factor that regulates cellular stress responses. While the levels of HIF-1alpha protein are tightly regulated, recent studies suggest that it can be active under normoxic conditions. We hypothesized that HIF-1alpha is required for normal beta cell function and reserve and that dysregulation may contribute to the pathogenesis of type 2 diabetes (T2D). Here we show that HIF-1alpha protein is present at low levels in mouse and human normoxic beta cells and islets. Decreased levels of HIF-1alpha impaired glucose-stimulated ATP generation and beta cell function. C57BL/6 mice with beta cell-specific Hif1a disruption (referred to herein as beta-Hif1a-null mice) exhibited glucose intolerance, beta cell dysfunction, and developed severe glucose intolerance on a high-fat diet. Increasing HIF-1alpha levels by inhibiting its degradation through iron chelation markedly improved insulin secretion and glucose tolerance in control mice fed a high-fat diet but not in beta-Hif1a-null mice. Increasing HIF-1alpha levels markedly increased expression of ARNT and other genes in human T2D islets and improved their function. Further analysis indicated that HIF-1alpha was bound to the Arnt promoter in a mouse beta cell line, suggesting direct regulation. Taken together, these findings suggest an important role for HIF-1alpha in beta cell reserve and regulation of ARNT expression and demonstrate that HIF-1alpha is a potential therapeutic target for the beta cell dysfunction of T2D.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

