Trafficking of immune cells in the central nervous system
- PMID: 20440079
- PMCID: PMC2860945
- DOI: 10.1172/JCI41911
Trafficking of immune cells in the central nervous system
Abstract
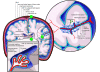
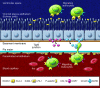
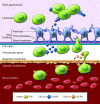
The CNS is an immune-privileged environment, yet the local control of multiple pathogens is dependent on the ability of immune cells to access and operate within this site. However, inflammation of the distinct anatomical sites (i.e., meninges, cerebrospinal fluid, and parenchyma) associated with the CNS can also be deleterious. Therefore, control of lymphocyte entry and migration within the brain is vital to regulate protective and pathological responses. In this review, several recent advances are highlighted that provide new insights into the processes that regulate leukocyte access to, and movement within, the brain.
Figures
References
-
- Ehrlich P.Das sauerstufbudurfnis des organismus. Eine Farbenanalytische Studie . Berlin, Germany: Hirschwald; 1885.
-
- Goldmann EE. Vitalfarbung am zentralnervensystem. Abhandl Konigl preuss Akad Wiss. 1913;1:1–60.
-
- Ge S, Song L, Serwanski DR, Kuziel WA, Pachter JS. Transcellular transport of CCL2 across brain microvascular endothelial cells. J Neurochem. 2008;104(5):1219–1232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

