Hepatic stellate cells' involvement in progenitor-mediated liver regeneration
- PMID: 20440274
- PMCID: PMC2912420
- DOI: 10.1038/labinvest.2010.88
Hepatic stellate cells' involvement in progenitor-mediated liver regeneration
Abstract
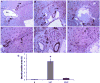
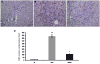
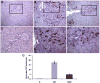
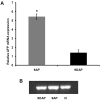
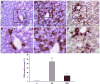
Earlier studies conducted by our laboratory have shown that suppression of transforming growth factor-beta (TGFbeta)-mediated upregulation of connective tissue growth factor (CTGF) by iloprost resulted in a greatly diminished oval cell response to 2-acetylaminofluorene/partial hepatectomy (2AAF/PH) in rats. We hypothesized that this effect is due to decreased activation of hepatic stellate cells. To test this hypothesis, we maintained rats on a diet supplemented with 2% L-cysteine as a means of inhibiting stellate cell activation during the oval cell response to 2AAF/PH. In vitro experiments show that L-cysteine did, indeed, prevent the activation of stellate cells while exerting no direct effect on oval cells. Desmin immunostaining of liver sections from 2AAF/PH animals indicated that maintenance on the L-cysteine diet resulted in an 11.1-fold decrease in the number of activated stellate cells within the periportal zones. The total number of cells proliferating in the periportal zones of livers from animals treated with L-cysteine was drastically reduced. Further analyses showed a greater than fourfold decrease in the magnitude of the oval cell response in animals maintained on the L-cysteine diet as determined by immunostaining for both OV6 and alpha-fetoprotein (AFP). Global liver expression of AFP as measured by real-time PCR was shown to be decreased 4.7-fold in the L-cysteine-treated animals. These data indicate that the activation of hepatic stellate cells is required for an appropriate oval cell response to 2AAF/PH.
Figures
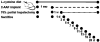




References
-
- Friedman SL. Liver fibrosis – from bench to bedside. J Hepatol. 2003;38(Suppl 1):S38–S53. - PubMed
-
- Ujike K, Shinji T, Hirasaki S, Shiraha H, Nakamura M, Tsuji T, Koide N. Kinetics of expression of connective tissue growth factor gene during liver regeneration after partial hepatectomy and D-galactosamine-induced liver injury in rats. Biochem Biophys Res Commun. 2000;277:448–454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

