Functionality of unliganded VDR in breast cancer cells: repressive action on CYP24 basal transcription
- PMID: 20440542
- PMCID: PMC2923673
- DOI: 10.1007/s11010-010-0478-6
Functionality of unliganded VDR in breast cancer cells: repressive action on CYP24 basal transcription
Abstract
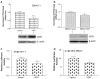
It is well-established that CYP24, an immediate target gene of VDR is upregulated by VDR ligands. This study is focused on the functional role of unliganded VDR by investigating the correlation between the expression of VDR protein and basal mRNA levels of CYP24 in breast cancer cell lines. Analyses of multiple breast cancer cell lines demonstrated an inverse correlation between VDR protein expression and CYP24 mRNA expression levels; while in the presence of ligand, VDR protein level was positively correlated with CYP24 expression. In MCF-7 cells, VDR was mainly distributed in the nuclei in the absence of ligand. VDR overexpression in MCF-7 cells and MDA-MB231 cells decreased CYP24 mRNA expression levels and CYP24 promoter activity. Conversely, knock-down of VDR using siRNA techniques in MCF-7 and T47D cells significantly increased CYP24 mRNA expression. We also found that overexpression of VDR with a polymorphic site (FokI-FF) at its AF-1 domain, which makes VDR shorter by three amino acids, failed to repress CYP24 promoter activity. This report provides conclusive evidence for the repressive action of unliganded VDR on the expression of its target gene CYP24 and the importance of an intact VDR AF-1 domain for its repressive action.
Figures



References
-
- Hewison M, Dabrowski M, Vadher S, Faulkner L, Cockerill FJ, Brickell PM, O’Riordan JL, Katz DR. Antisense inhibition of vitamin D receptor expression induces apoptosis in monoblastoid U937 cells. J Immunol. 1996;156:4391–4400. - PubMed
-
- Guzey M, Jukic D, Arlotti J, Acquafondata M, Dhir R, Getzenberg RH. Increased apoptosis of periprostatic adipose tissue in VDR null mice. J Cell Biochem. 2004;93:133–141. - PubMed
-
- Blumberg JM, Tzameli I, Astapova I, Lam FS, Flier JS, Hollenberg AN. Complex role of the vitamin D receptor and its ligand in adipogenesis in 3T3-L1 cells. J Biol Chem. 2006;281:11205–11213. - PubMed
-
- Prüfer K, Barsony J. Retinoid X receptor dominates the nuclear import and export of the unliganded vitamin D receptor. Mol Endocrinol. 2006;16:1738–1751. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

