Size and dynamics of the Vibrio cholerae porins OmpU and OmpT probed by polymer exclusion
- PMID: 20441745
- PMCID: PMC2862161
- DOI: 10.1016/j.bpj.2010.01.010
Size and dynamics of the Vibrio cholerae porins OmpU and OmpT probed by polymer exclusion
Abstract
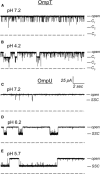
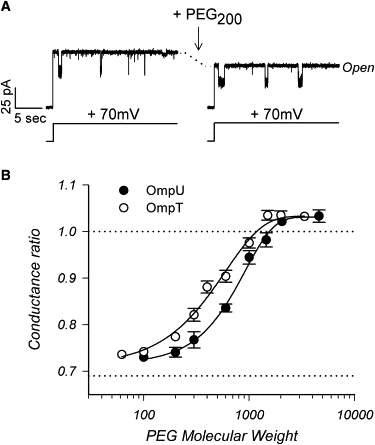
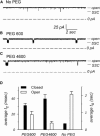
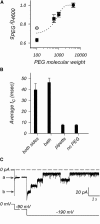
The trimeric OmpU and OmpT porins form large, triple-barrel hydrophilic channels in the outer membrane of the pathogen Vibrio cholerae. They have distinct pore properties, such as conductance, block by deoxycholic acid, and sensitivity to acidic pH. Their three-dimensional structures are unknown, but they share significant sequence homologies. To gain insight into the molecular basis for the distinct functional properties of these two similar porins, we carried out polymer exclusion experiments using planar lipid bilayer and patch-clamp electrophysiology. By studying the partitioning of polyethylene glycols (PEGs) of different molecular weights into each porin, we determined an effective radius of 0.55 nm and 0.43 nm for OmpU and OmpT respectively, and found an increased OmpU effective radius at acidic pH. PEGs or high buffer ionic strength promotes the appearance of single step closures in OmpU similar to the acidic-pH induced closures we documented previously. In addition, these closing events can be triggered by nonpenetrating PEGs applied asymmetrically. We believe our results support a model whereby acidic pH, high ionic strength, or exposure to PEGs stabilizes a less conductive state that corresponds to the appearance of an additional resistive element on one side of the OmpU protein and common to the three monomers.
Copyright (c) 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
The Vibrio cholerae porins OmpU and OmpT have distinct channel properties.J Biol Chem. 2003 May 9;278(19):17539-45. doi: 10.1074/jbc.M301202200. Epub 2003 Feb 25. J Biol Chem. 2003. PMID: 12606562
-
Unusual Constriction Zones in the Major Porins OmpU and OmpT from Vibrio cholerae.Structure. 2018 May 1;26(5):708-721.e4. doi: 10.1016/j.str.2018.03.010. Epub 2018 Apr 12. Structure. 2018. PMID: 29657131
-
Characterization of the role of the ToxR-modulated outer membrane porins OmpU and OmpT in Vibrio cholerae virulence.J Bacteriol. 2001 Jun;183(12):3652-62. doi: 10.1128/JB.183.12.3652-3662.2001. J Bacteriol. 2001. PMID: 11371530 Free PMC article.
-
Mycobacterial porins--new channel proteins in unique outer membranes.Mol Microbiol. 2003 Sep;49(5):1167-77. doi: 10.1046/j.1365-2958.2003.03662.x. Mol Microbiol. 2003. PMID: 12940978 Review.
-
Voltage gating is a fundamental feature of porin and toxin beta-barrel membrane channels.FEBS Lett. 1998 Jul 24;431(3):305-8. doi: 10.1016/s0014-5793(98)00761-3. FEBS Lett. 1998. PMID: 9714531 Review.
Cited by
-
Effects of amino acid supplementation on porin expression and ToxR levels in Vibrio cholerae.Infect Immun. 2012 Feb;80(2):518-28. doi: 10.1128/IAI.05851-11. Epub 2011 Dec 5. Infect Immun. 2012. PMID: 22144480 Free PMC article.
-
Identification of a pore-forming protein from sea anemone Anthopleura dowii Verrill (1869) venom by mass spectrometry.J Venom Anim Toxins Incl Trop Dis. 2019 Feb 11;25:e147418. doi: 10.1590/1678-9199-JVATITD-1474-18. eCollection 2019. J Venom Anim Toxins Incl Trop Dis. 2019. PMID: 31131002 Free PMC article.
-
Positive regulation of the Vibrio cholerae porin OmpT by iron and fur.J Bacteriol. 2011 Dec;193(23):6505-11. doi: 10.1128/JB.05681-11. Epub 2011 Sep 30. J Bacteriol. 2011. PMID: 21965571 Free PMC article.
-
Vibrio cholerae LeuO Links the ToxR Regulon to Expression of Lipid A Remodeling Genes.Infect Immun. 2016 Oct 17;84(11):3161-3171. doi: 10.1128/IAI.00445-16. Print 2016 Nov. Infect Immun. 2016. PMID: 27550934 Free PMC article.
-
Crystal structure of the outer membrane protein OmpU from Vibrio cholerae at 2.2 Å resolution.Acta Crystallogr D Struct Biol. 2018 Jan 1;74(Pt 1):21-29. doi: 10.1107/S2059798317017697. Epub 2018 Jan 1. Acta Crystallogr D Struct Biol. 2018. PMID: 29372896 Free PMC article.
References
-
- Benz R., Janko K., Läuger P. Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim. Biophys. Acta. 1978;511:305–319. - PubMed
-
- Delcour A.H. Solute uptake through general porins. Front. Biosci. 2003;8:d1055–d1071. - PubMed
-
- Baslé A., Rummel G., Schirmer T. Crystal structure of osmoporin OmpC from E. coli at 2.0 Å. J. Mol. Biol. 2006;362:933–942. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

