Urea facilitates the translocation of single-stranded DNA and RNA through the alpha-hemolysin nanopore
- PMID: 20441749
- PMCID: PMC2862201
- DOI: 10.1016/j.bpj.2009.12.4333
Urea facilitates the translocation of single-stranded DNA and RNA through the alpha-hemolysin nanopore
Abstract
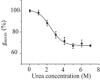
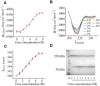
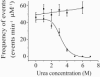
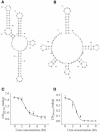
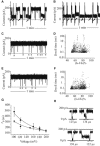
The staphylococcal alpha-hemolysin (alphaHL) protein nanopore is under investigation as a fast, cheap detector for nucleic acid analysis and sequencing. Although discrimination of all four bases of DNA by the alphaHL pore has been demonstrated, analysis of single-stranded DNAs and RNAs containing secondary structure mediated by basepairing is prevented because these nucleic acids cannot be translocated through the pore. Here, we show that a structured 95-nucleotide single-stranded DNA and its RNA equivalent are translocated through the alphaHL pore in the presence of 4 M urea, a concentration that denatures the secondary structure of the polynucleotides. The alphaHL pore is functional even in 7 M urea, and therefore it is easily stable enough for analyses of challenging DNA and RNA species.
Copyright (c) 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
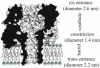
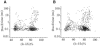
Similar articles
-
Unzipping of A-Form DNA-RNA, A-Form DNA-PNA, and B-Form DNA-DNA in the α-Hemolysin Nanopore.Biophys J. 2016 Jan 19;110(2):306-314. doi: 10.1016/j.bpj.2015.11.020. Biophys J. 2016. PMID: 26789754 Free PMC article.
-
Voltage-driven DNA translocations through a nanopore.Phys Rev Lett. 2001 Apr 9;86(15):3435-8. doi: 10.1103/PhysRevLett.86.3435. Phys Rev Lett. 2001. PMID: 11327989
-
Impact of distant charge reversals within a robust beta-barrel protein pore.J Phys Chem B. 2010 Jul 8;114(26):8750-9. doi: 10.1021/jp101311s. J Phys Chem B. 2010. PMID: 20540583 Free PMC article.
-
Staphylococcal pore-forming toxins.Curr Top Microbiol Immunol. 2001;257:53-83. doi: 10.1007/978-3-642-56508-3_4. Curr Top Microbiol Immunol. 2001. PMID: 11417122 Review. No abstract available.
-
Characterization of nucleic acids by nanopore analysis.Acc Chem Res. 2002 Oct;35(10):817-25. doi: 10.1021/ar000138m. Acc Chem Res. 2002. PMID: 12379134 Review.
Cited by
-
Crown ether-electrolyte interactions permit nanopore detection of individual DNA abasic sites in single molecules.Proc Natl Acad Sci U S A. 2012 Jul 17;109(29):11504-9. doi: 10.1073/pnas.1201669109. Epub 2012 Jun 18. Proc Natl Acad Sci U S A. 2012. PMID: 22711805 Free PMC article.
-
Applications and potentials of nanopore sequencing in the (epi)genome and (epi)transcriptome era.Innovation (Camb). 2021 Aug 11;2(4):100153. doi: 10.1016/j.xinn.2021.100153. eCollection 2021 Nov 28. Innovation (Camb). 2021. PMID: 34901902 Free PMC article. Review.
-
Differentiation of G:C vs A:T and G:C vs G:mC Base Pairs in the Latch Zone of α-Hemolysin.ACS Nano. 2015 Nov 24;9(11):11325-32. doi: 10.1021/acsnano.5b05055. Epub 2015 Oct 27. ACS Nano. 2015. PMID: 26506108 Free PMC article.
-
Fabrication and Applications of Solid-State Nanopores.Sensors (Basel). 2019 Apr 20;19(8):1886. doi: 10.3390/s19081886. Sensors (Basel). 2019. PMID: 31010038 Free PMC article. Review.
-
Computational studies of DNA sequencing with solid-state nanopores: key issues and future prospects.Front Chem. 2014 Feb 21;2:5. doi: 10.3389/fchem.2014.00005. eCollection 2014. Front Chem. 2014. PMID: 24790974 Free PMC article. Review.
References
-
- Song L., Hobaugh M.R., Gouaux J.E. Structure of staphylococcal α-hemolysin, a heptameric transmembrane pore. Science. 1996;274:1859–1866. - PubMed
-
- Henrickson S.E., Misakian M., Kasianowicz J.J. Driven DNA transport into an asymmetric nanometer-scale pore. Phys. Rev. Lett. 2000;85:3057–3060. - PubMed
-
- Kasianowicz J.J., Henrickson S.E., Robertson B. Simultaneous multianalyte detection with a nanometer-scale pore. Anal. Chem. 2001;73:2268–2272. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

