Phosphoenolpyruvate and Mg2+ binding to pyruvate kinase monitored by infrared spectroscopy
- PMID: 20441757
- PMCID: PMC2862152
- DOI: 10.1016/j.bpj.2009.12.4335
Phosphoenolpyruvate and Mg2+ binding to pyruvate kinase monitored by infrared spectroscopy
Abstract
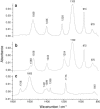
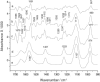
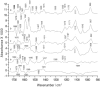
Structural changes in rabbit muscle pyruvate kinase (PK) induced by phosphoenolpyruvate (PEP) and Mg(2+) binding were studied by attenuated total reflection Fourier transform infrared spectroscopy in combination with a dialysis accessory. The experiments indicated a largely preserved secondary structure upon PEP and Mg(2+) binding but also revealed small backbone conformational changes of PK involving all types of secondary structure. To assess the effect of the protein environment on the bound PEP, we assigned and evaluated the infrared absorption bands of bound PEP. These were identified using 2,3-(13)C(2)-labeled PEP. We obtained the following assignments: 1589 cm(-1) (antisymmetric carboxylate stretching vibration); 1415 cm(-1) (symmetric carboxylate stretching vibration); 1214 cm(-1) (C-O stretching vibration); 1124 and 1110 cm(-1) (asymmetric PO(3)(2-) stretching vibrations); and 967 cm(-1) (symmetric PO(3)(2-) stretching vibration). The corresponding band positions in solution are 1567, 1407, 1229, 1107, and 974 cm(-1). The differences for bound and free PEP indicate specific interactions between ligand and protein. Quantification of the interactions with the phosphate group indicated that the enzyme environment has little influence on the P-O bond strengths, and that the bridging P-O bond, which is broken in the catalytic reaction, is weakened by <3%. Thus, there is only little distortion toward a dissociative transition state of the phosphate transfer reaction when PEP binds to PK. Therefore, our results are in line with an associative transition state. Carboxylate absorption bands indicated a maximal shortening of the length of the shorter C-O bond by 1.3 pm. PEP bound to PK in the presence of the monovalent ion Na(+) exhibited the same band positions as in the presence of K(+), indicating very similar interaction strengths between ligand and protein in both cases.
Copyright (c) 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Effects of ions on ligand binding to pyruvate kinase: mapping the binding site with infrared spectroscopy.J Phys Chem B. 2011 May 26;115(20):6784-9. doi: 10.1021/jp201862a. Epub 2011 May 3. J Phys Chem B. 2011. PMID: 21539324
-
The allosteric effect of fructose bisphosphate on muscle pyruvate kinase studied by infrared spectroscopy.J Phys Chem B. 2011 Oct 6;115(39):11501-5. doi: 10.1021/jp206272x. Epub 2011 Sep 9. J Phys Chem B. 2011. PMID: 21870844
-
Effects of metabolites on the structural dynamics of rabbit muscle pyruvate kinase.Biophys Chem. 2003 Jan 8;103(1):1-11. doi: 10.1016/s0301-4622(02)00146-1. Biophys Chem. 2003. PMID: 12504250
-
Detection of ligand binding to proteins through observation of hydration water.J Phys Chem B. 2012 Dec 6;116(48):13968-74. doi: 10.1021/jp307560r. Epub 2012 Nov 19. J Phys Chem B. 2012. PMID: 23151018
-
Synthesis of phosphoenol pyruvate (PEP) analogues and evaluation as inhibitors of PEP-utilizing enzymes.Eur J Biochem. 2002 Jul;269(13):3226-36. doi: 10.1046/j.1432-1033.2002.02995.x. Eur J Biochem. 2002. PMID: 12084063
Cited by
-
RNA-Seq transcriptome analysis of ileum in Taiping chicken supplemented with the dietary probiotic.Trop Anim Health Prod. 2021 Jan 19;53(1):131. doi: 10.1007/s11250-021-02566-w. Trop Anim Health Prod. 2021. PMID: 33462736
-
The PEP-pyruvate-oxaloacetate node: variation at the heart of metabolism.FEMS Microbiol Rev. 2021 May 5;45(3):fuaa061. doi: 10.1093/femsre/fuaa061. FEMS Microbiol Rev. 2021. PMID: 33289792 Free PMC article.
-
Biophysical Approaches for the Characterization of Protein-Metabolite Interactions.Methods Mol Biol. 2023;2554:199-229. doi: 10.1007/978-1-0716-2624-5_13. Methods Mol Biol. 2023. PMID: 36178628 Review.
-
Following enzyme activity with infrared spectroscopy.Sensors (Basel). 2010;10(4):2626-37. doi: 10.3390/s100402626. Epub 2010 Mar 25. Sensors (Basel). 2010. PMID: 22319264 Free PMC article.
-
A short review on cross-link between pyruvate kinase (PKM2) and Glioblastoma Multiforme.Metab Brain Dis. 2021 Jun;36(5):751-765. doi: 10.1007/s11011-021-00690-y. Epub 2021 Mar 2. Metab Brain Dis. 2021. PMID: 33651273 Review.
References
-
- Seeholzer S.H., Jaworowski A., Rose I.A. Enolpyruvate: chemical determination as a pyruvate kinase intermediate. Biochemistry. 1991;30:727–732. - PubMed
-
- Rose I.A. Stereochemistry of pyruvate kinase, pyruvate carboxylase, and malate enzyme reactions. J. Biol. Chem. 1970;245:6052–6056. - PubMed
-
- Larsen T.M., Benning M.M., Reed G.H. Structure of the bis(Mg2+)-ATP-oxalate complex of the rabbit muscle pyruvate kinase at 2.1 Å resolution: ATP binding over a barrel. Biochemistry. 1998;37:6247–6255. - PubMed
-
- Larsen T.M., Benning M.M., Reed G.H. Ligand-induced domain movement in pyruvate kinase: structure of the enzyme from rabbit muscle with Mg2+, K+, and L-phospholactate at 2.7 Å resolution. Arch. Biochem. Biophys. 1997;345:199–206. - PubMed
-
- Gupta R.K., Oesterling R.M., Mildvan A.S. Dual divalent cation requirement for activation of pyruvate kinase; essential roles of both enzyme- and nucleotide-bound metal ions. Biochemistry. 1976;15:2881–2887. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

