Fragile X mental retardation protein has a unique, evolutionarily conserved neuronal function not shared with FXR1P or FXR2P
- PMID: 20442204
- PMCID: PMC2898537
- DOI: 10.1242/dmm.004598
Fragile X mental retardation protein has a unique, evolutionarily conserved neuronal function not shared with FXR1P or FXR2P
Abstract
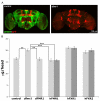
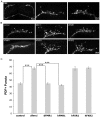
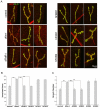
Fragile X syndrome (FXS), resulting solely from the loss of function of the human fragile X mental retardation 1 (hFMR1) gene, is the most common heritable cause of mental retardation and autism disorders, with syndromic defects also in non-neuronal tissues. In addition, the human genome encodes two closely related hFMR1 paralogs: hFXR1 and hFXR2. The Drosophila genome, by contrast, encodes a single dFMR1 gene with close sequence homology to all three human genes. Drosophila that lack the dFMR1 gene (dfmr1 null mutants) recapitulate FXS-associated molecular, cellular and behavioral phenotypes, suggesting that FMR1 function has been conserved, albeit with specific functions possibly sub-served by the expanded human gene family. To test evolutionary conservation, we used tissue-targeted transgenic expression of all three human genes in the Drosophila disease model to investigate function at (1) molecular, (2) neuronal and (3) non-neuronal levels. In neurons, dfmr1 null mutants exhibit elevated protein levels that alter the central brain and neuromuscular junction (NMJ) synaptic architecture, including an increase in synapse area, branching and bouton numbers. Importantly, hFMR1 can, comparably to dFMR1, fully rescue both the molecular and cellular defects in neurons, whereas hFXR1 and hFXR2 provide absolutely no rescue. For non-neuronal requirements, we assayed male fecundity and testes function. dfmr1 null mutants are effectively sterile owing to disruption of the 9+2 microtubule organization in the sperm tail. Importantly, all three human genes fully and equally rescue mutant fecundity and spermatogenesis defects. These results indicate that FMR1 gene function is evolutionarily conserved in neural mechanisms and cannot be compensated by either FXR1 or FXR2, but that all three proteins can substitute for each other in non-neuronal requirements. We conclude that FMR1 has a neural-specific function that is distinct from its paralogs, and that the unique FMR1 function is responsible for regulating neuronal protein expression and synaptic connectivity.
Figures

Similar articles
-
In vivo neuronal function of the fragile X mental retardation protein is regulated by phosphorylation.Hum Mol Genet. 2012 Feb 15;21(4):900-15. doi: 10.1093/hmg/ddr527. Epub 2011 Nov 11. Hum Mol Genet. 2012. PMID: 22080836 Free PMC article.
-
Neural circuit architecture defects in a Drosophila model of Fragile X syndrome are alleviated by minocycline treatment and genetic removal of matrix metalloproteinase.Dis Model Mech. 2011 Sep;4(5):673-85. doi: 10.1242/dmm.008045. Epub 2011 Jun 13. Dis Model Mech. 2011. PMID: 21669931 Free PMC article.
-
Argonaute2 suppresses Drosophila fragile X expression preventing neurogenesis and oogenesis defects.PLoS One. 2009 Oct 27;4(10):e7618. doi: 10.1371/journal.pone.0007618. PLoS One. 2009. PMID: 19888420 Free PMC article.
-
dFmr1 Plays Roles in Small RNA Pathways of Drosophila melanogaster.Int J Mol Sci. 2017 May 16;18(5):1066. doi: 10.3390/ijms18051066. Int J Mol Sci. 2017. PMID: 28509881 Free PMC article. Review.
-
Modeling Fragile X Syndrome in Drosophila.Front Mol Neurosci. 2018 Apr 16;11:124. doi: 10.3389/fnmol.2018.00124. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29713264 Free PMC article. Review.
Cited by
-
Regulation of Adult Neurogenesis by the Fragile X Family of RNA Binding Proteins.Brain Plast. 2018 Aug 10;3(2):205-223. doi: 10.3233/BPL-170061. Brain Plast. 2018. PMID: 30151344 Free PMC article. Review.
-
Fragile X Mental Retardation Protein Regulates Activity-Dependent Membrane Trafficking and Trans-Synaptic Signaling Mediating Synaptic Remodeling.Front Mol Neurosci. 2018 Jan 12;10:440. doi: 10.3389/fnmol.2017.00440. eCollection 2017. Front Mol Neurosci. 2018. PMID: 29375303 Free PMC article. Review.
-
Fragile X mental retardation protein is required for programmed cell death and clearance of developmentally-transient peptidergic neurons.Dev Biol. 2011 Aug 15;356(2):291-307. doi: 10.1016/j.ydbio.2011.05.001. Epub 2011 May 10. Dev Biol. 2011. PMID: 21596027 Free PMC article.
-
Structural and Functional Abnormalities in the Olfactory System of Fragile X Syndrome Models.Front Mol Neurosci. 2019 May 28;12:135. doi: 10.3389/fnmol.2019.00135. eCollection 2019. Front Mol Neurosci. 2019. PMID: 31191246 Free PMC article. Review.
-
Exposure to bisphenol A differentially impacts neurodevelopment and behavior in Drosophila melanogaster from distinct genetic backgrounds.Neurotoxicology. 2021 Jan;82:146-157. doi: 10.1016/j.neuro.2020.12.007. Epub 2020 Dec 10. Neurotoxicology. 2021. PMID: 33309840 Free PMC article.
References
-
- Agulhon C, Blanchet P, Kobetz A, Marchant D, Faucon N, Sarda P, Moraine C, Sittler A, Biancalana V, Malafosse A, et al. (1999). Expression of FMR1, FXR1, and FXR2 genes in human prenatal tissues. J Neuropathol Exp Neurol. 58, 867–880 - PubMed
-
- Bakker CE, de Diego Otero Y, Bontekoe C, Raghoe P, Luteijn T, Hoogeveen AT, Oostra BA, Willemsen R. (2000). Immunocytochemical and biochemical characterization of FMRP, FXR1P, and FXR2P in the mouse. Exp Cell Res. 258, 162–170 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

