Endogenous endothelin stimulates cardiac sympathetic afferents during ischaemia
- PMID: 20442267
- PMCID: PMC2915521
- DOI: 10.1113/jphysiol.2010.188730
Endogenous endothelin stimulates cardiac sympathetic afferents during ischaemia
Abstract
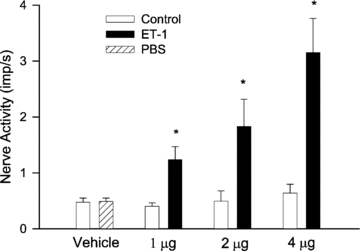
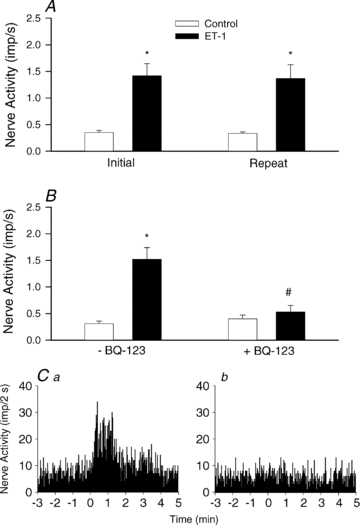
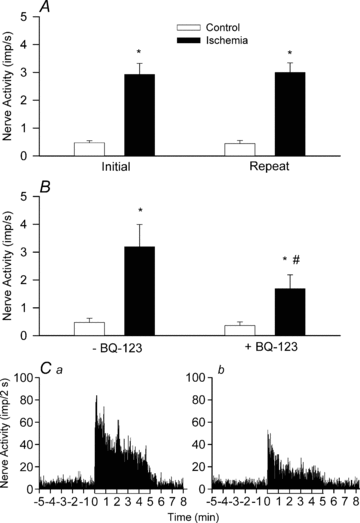
Myocardial ischaemia activates cardiac sympathetic afferents leading to chest pain and reflex cardiovascular responses. Previous studies have shown that a brief period of myocardial ischaemia increases endothelin in cardiac venous plasma draining ischaemic myocardium and that exogenous endothelin excites cutaneous group III and IV sensory nerve fibres. The present study tested the hypothesis that endogenous endothelin stimulates cardiac afferents during ischaemia through direct activation of endothelin A receptors (ET(A)Rs). Nerve activity of single unit cardiac sympathetic afferents was recorded from the left sympathetic chain or rami communicates (T(2)-T(5)) in anaesthetized cats. Single fields of 38 afferents (CV = 0.25-3.86 m s(-1)) were identified in the left or right ventricle with a stimulating electrode. Five minutes of myocardial ischaemia stimulated all 38 cardiac afferents (8 Adelta, 30 C-fibres) and the responses of these 38 afferents to chemical stimuli were further studied in the following protocols. In the first protocol, injection of endothelin 1 (ET-1, 1, 2 and 4 microg) into the left atrium (LA) stimulated seven ischaemically sensitive cardiac afferents in a dose-dependent manner. Second, BQ-123, a selective ET(A)R antagonist, abolished the responses of nine afferents to 2 microg of ET-1 injected into the left atrium and attenuated the ischaemia-related increase in activity of eight other afferents by 51%. In contrast, blockade of ET(B) receptors caused inconsistent responses to exogenous ET-1 as well as to ischaemia. Furthermore, in the absence of ET(A)R blockade, cardiac afferents responded consistently to repeated administration of ET-1 (n = 7) and to recurrent myocardial ischaemia (n = 7). Finally, using an immunocytochemical staining approach, we observed that ET(A) receptors were expressed in cardiac sensory neurons in thoracic dorsal root ganglia. Taken together, these data indicate that endogenous endothelin contributes to activation of cardiac afferents during myocardial ischaemia through direct stimulation of ET(A) receptors likely to be located in the cardiac sensory nervous system.
Figures


Similar articles
-
A new function for ATP: activating cardiac sympathetic afferents during myocardial ischemia.Am J Physiol Heart Circ Physiol. 2010 Dec;299(6):H1762-71. doi: 10.1152/ajpheart.00822.2010. Epub 2010 Sep 24. Am J Physiol Heart Circ Physiol. 2010. PMID: 20870803 Free PMC article.
-
Undiscovered role of endogenous thromboxane A2 in activation of cardiac sympathetic afferents during ischaemia.J Physiol. 2008 Jul 1;586(13):3287-300. doi: 10.1113/jphysiol.2007.148106. Epub 2008 May 15. J Physiol. 2008. PMID: 18483073 Free PMC article.
-
Activated platelets contribute to stimulation of cardiac afferents during ischaemia in cats: role of 5-HT(3) receptors.J Physiol. 2002 Nov 1;544(3):897-912. doi: 10.1113/jphysiol.2002.023374. J Physiol. 2002. PMID: 12411532 Free PMC article.
-
Reflexes mediated by cardiac sympathetic afferents during myocardial ischaemia: role of adenosine.Clin Exp Pharmacol Physiol. 1996 Aug;23(8):709-14. doi: 10.1111/j.1440-1681.1996.tb01763.x. Clin Exp Pharmacol Physiol. 1996. PMID: 8886495 Review.
-
Cardiac sympathetic afferent activation provoked by myocardial ischemia and reperfusion. Mechanisms and reflexes.Ann N Y Acad Sci. 2001 Jun;940:74-95. doi: 10.1111/j.1749-6632.2001.tb03668.x. Ann N Y Acad Sci. 2001. PMID: 11458709 Review.
Cited by
-
Role of TRPV1 in acupuncture modulation of reflex excitatory cardiovascular responses.Am J Physiol Regul Integr Comp Physiol. 2018 May 1;314(5):R655-R666. doi: 10.1152/ajpregu.00405.2017. Epub 2018 Jan 3. Am J Physiol Regul Integr Comp Physiol. 2018. PMID: 29351423 Free PMC article.
-
A new function for ATP: activating cardiac sympathetic afferents during myocardial ischemia.Am J Physiol Heart Circ Physiol. 2010 Dec;299(6):H1762-71. doi: 10.1152/ajpheart.00822.2010. Epub 2010 Sep 24. Am J Physiol Heart Circ Physiol. 2010. PMID: 20870803 Free PMC article.
-
N-acetylcysteine treatment following spinal cord trauma reduces neural tissue damage and improves locomotor function in mice.Mol Med Rep. 2015 Jul;12(1):37-44. doi: 10.3892/mmr.2015.3390. Epub 2015 Feb 26. Mol Med Rep. 2015. PMID: 25738883 Free PMC article.
-
Low esophageal mucosal blood flow in patients with nutcracker esophagus.Am J Physiol Gastrointest Liver Physiol. 2016 Mar 15;310(6):G410-6. doi: 10.1152/ajpgi.00359.2015. Epub 2015 Dec 23. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 26702139 Free PMC article.
-
Cardiac Sympathetic Afferent Denervation Protects Against Ventricular Arrhythmias by Modulating Cardiac Sympathetic Nerve Activity During Acute Myocardial Infarction.Med Sci Monit. 2019 Mar 16;25:1984-1993. doi: 10.12659/MSM.914105. Med Sci Monit. 2019. PMID: 30877783 Free PMC article.
References
-
- Baamonde A, Lastra A, Villazon M, Bordallo J, Hidalgo A, Menendez L. Involvement of endogenous endothelins in thermal and mechanical inflammatory hyperalgesia in mice. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:245–251. - PubMed
-
- Balonov K, Khodorova A, Strichartz GR. Tactile allodynia initiated by local subcutaneous endothelin-1 is prolonged by activation of TRPV-1 receptors. Exp Biol Med. 2006;231:1165–1170. - PubMed
-
- Black SM, Mata-Greenwood E, Dettman RW, Ovadia B, Fitzgerald RK, Reinhartz O, Thelitz S, Steinhorn RH, Gerrets R, Hendricks-Munoz K, Ross GA, Bekker JM, Johengen MJ, Fineman JR. Emergence of smooth muscle cell endothelin B-mediated vasoconstriction in lambs with experimental congenital heart disease and increased pulmonary blood flow. Circulation. 2003;108:1646–1654. - PubMed
-
- Bohm F, Pernow J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc Res. 2007;76:8–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

