Thrombin regulates the metastatic potential of human rhabdomyosarcoma cells: distinct role of PAR1 and PAR3 signaling
- PMID: 20442298
- PMCID: PMC2896479
- DOI: 10.1158/1541-7786.MCR-10-0019
Thrombin regulates the metastatic potential of human rhabdomyosarcoma cells: distinct role of PAR1 and PAR3 signaling
Abstract
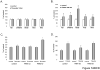
We observed that human rhabdomyosarcoma (RMS) cells highly express a tissue factor that promotes thrombin formation, which indirectly and directly affects RMS progression. First, we found that thrombin activates platelets to generate microvesicles (PMV), which transfer to RMS cells' alpha2beta3 integrin and increase their adhesiveness to endothelial cells. Accordingly, RMS cells covered with PMVs showed higher metastatic potential after i.v. injection into immunodeficient mice. Furthermore, PMVs activate mitogen-activated protein kinase (MAPK)p42/44 and AKT to chemoattract RMS cells. We also found that RMS cells express functional protease-activated receptor-1 (PAR1) and PAR3 and respond to thrombin stimulation by MAPKp42/44 and MAPKp38 phosphorylation. To our surprise, thrombin did not affect RMS proliferation or survival; it inhibited the chemotactic and adhesive properties of RMS cells. However, when PAR1-specific agonist thrombin receptor-activating peptide 6 was used, which does not activate PAR3, selective PAR1 stimulation enhanced RMS proliferation. To learn more on the role of PAR1 and PAR3 antagonism in RMS proliferation and metastasis, we knocked down both receptors by using a short hairpin RNA strategy. We found that although thrombin does not affect growth of PAR1(-/-) cells, it stimulated the proliferation of PAR3(-/-) cells. More importantly, PAR3(-/-) cells, in contrast to PAR1(-/-) ones, formed larger tumors in immunodeficient mice. We conclude that thrombin is a novel underappreciated modulator of RMS metastasis and that we have identified a novel role for PAR3 in thrombin signaling.
(c)2010 AACR.
Figures








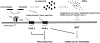
Similar articles
-
Protease-activated receptor-3 (PAR3) regulates PAR1 signaling by receptor dimerization.Proc Natl Acad Sci U S A. 2007 Mar 27;104(13):5662-7. doi: 10.1073/pnas.0700763104. Epub 2007 Mar 21. Proc Natl Acad Sci U S A. 2007. PMID: 17376866 Free PMC article.
-
Characterization and functional activity of thrombin receptors in the human lens.Invest Ophthalmol Vis Sci. 2005 Mar;46(3):925-32. doi: 10.1167/iovs.04-0523. Invest Ophthalmol Vis Sci. 2005. PMID: 15728549
-
Proteinase-activated receptors (PARs)--the PAR3 Neo-N-terminal peptide TFRGAP interacts with PAR1.Regul Pept. 2005 Feb 15;125(1-3):61-6. doi: 10.1016/j.regpep.2004.07.032. Regul Pept. 2005. PMID: 15582715
-
How the protease thrombin talks to cells.Proc Natl Acad Sci U S A. 1999 Sep 28;96(20):11023-7. doi: 10.1073/pnas.96.20.11023. Proc Natl Acad Sci U S A. 1999. PMID: 10500117 Free PMC article. Review.
-
Protease-activated receptors in cardiovascular diseases.Circulation. 2006 Sep 5;114(10):1070-7. doi: 10.1161/CIRCULATIONAHA.105.574830. Circulation. 2006. PMID: 16952995 Review.
Cited by
-
GPCRs and cancer.Acta Pharmacol Sin. 2012 Mar;33(3):351-62. doi: 10.1038/aps.2011.183. Epub 2012 Jan 23. Acta Pharmacol Sin. 2012. PMID: 22266725 Free PMC article. Review.
-
Proteinase-activated receptors differentially modulate in vitro invasion of human pancreatic adenocarcinoma PANC-1 cells in correlation with changes in the expression of CDC42 protein.Pancreas. 2014 Jan;43(1):103-8. doi: 10.1097/MPA.0b013e31829f0b81. Pancreas. 2014. PMID: 23921961 Free PMC article.
-
Pathologies at the nexus of blood coagulation and inflammation: thrombin in hemostasis, cancer, and beyond.J Mol Med (Berl). 2013 Nov;91(11):1257-71. doi: 10.1007/s00109-013-1074-5. Epub 2013 Aug 17. J Mol Med (Berl). 2013. PMID: 23955016 Free PMC article. Review.
-
Does it make sense to target one tumor cell chemotactic factor or its receptor when several chemotactic axes are involved in metastasis of the same cancer?Clin Transl Med. 2016 Dec;5(1):28. doi: 10.1186/s40169-016-0113-6. Epub 2016 Aug 10. Clin Transl Med. 2016. PMID: 27510263 Free PMC article. Review.
-
Macrophage migration inhibitory factor is secreted by rhabdomyosarcoma cells, modulates tumor metastasis by binding to CXCR4 and CXCR7 receptors and inhibits recruitment of cancer-associated fibroblasts.Mol Cancer Res. 2010 Oct;8(10):1328-43. doi: 10.1158/1541-7786.MCR-10-0288. Epub 2010 Sep 22. Mol Cancer Res. 2010. PMID: 20861157 Free PMC article.
References
-
- Ruymann FB, Newton WA, Ragab AH, Donaldson MH, Foulkes M. Bone marrow metastases at diagnosis in children and adolescents with rhabdomyosarcoma. Cancer. 1984;53:368–373. - PubMed
-
- Dickman PS, Tsokos M, Triche TJ. Biology of rhabdomyosarcoma: cell culture, xenografts and animal models. In: Maurer HM, Ruymann FB, Pochedly C, editors. Rhabdomyosarcoma and Related Tumours in Children and Adolescents. Boca Raton, FL: CRC Press; 1991. pp. 49–88.
-
- Sandberg AA, Stone JF, Czarnecki L, Cohen JD. Hematologic masquerade of rhabdomyosarcoma. Am J Hematol. 2001;68:51–57. - PubMed
-
- Barr FG, Galili N, Holick J, Biegel JA, Rovera G, Emanuel BS. Rearrangement of the PAX3 paired box gene in the paediatric solid tumour alveolar rhabdomyosarcoma. Nat Genet. 1993;3:113–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

