An AP-3-dependent mechanism drives synaptic-like microvesicle biogenesis in pancreatic islet beta-cells
- PMID: 20442321
- PMCID: PMC2904044
- DOI: 10.1152/ajpendo.00664.2009
An AP-3-dependent mechanism drives synaptic-like microvesicle biogenesis in pancreatic islet beta-cells
Abstract
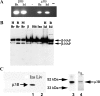
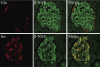
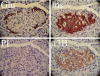
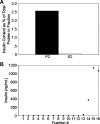
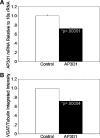
Pancreatic islet beta-cells contain synaptic-like microvesicles (SLMVs). The origin, trafficking, and role of these SLMVs are poorly understood. In neurons, synaptic vesicle (SV) biogenesis is mediated by two different cytosolic adaptor protein complexes, a ubiquitous AP-2 complex and the neuron-specific AP-3B complex. Mice lacking AP-3B subunits exhibit impaired GABAergic (inhibitory) neurotransmission and reduced neuronal vesicular GABA transporter (VGAT) content. Since beta-cell maturation and exocytotic function seem to parallel that of the inhibitory synapse, we predicted that AP-3B-associated vesicles would be present in beta-cells. Here, we test the hypothesis that AP-3B is expressed in islets and mediates beta-cell SLMV biogenesis. A secondary aim was to test whether the sedimentation properties of INS-1 beta-cell microvesicles are identical to those of bona fide SLMVs isolated from PC12 cells. Our results show that the two neuron-specific AP-3 subunits beta3B and mu3B are expressed in beta-cells, the first time these proteins have been found to be expressed outside the nervous system. We found that beta-cell SLMVs share the same sedimentation properties as PC12 SLMVs and contain SV proteins that sort specifically to AP-3B-associated vesicles in the brain. Brefeldin A, a drug that interferes with AP-3-mediated SV biogenesis, inhibits the delivery of AP-3 cargoes to beta-cell SLMVs. Consistent with a role for AP-3 in the biogenesis of GABAergic SLMV in beta-cells, INS-1 cell VGAT content decreases upon inhibition of AP-3 delta-subunit expression. Our findings suggest that beta-cells and neurons share molecules and mechanisms important for mediating the neuron-specific membrane trafficking pathways that underlie synaptic vesicle formation.
Figures


Similar articles
-
A role for synaptic vesicles in non-neuronal cells: clues from pancreatic beta cells and from chromaffin cells.FASEB J. 1994 Feb;8(2):209-16. doi: 10.1096/fasebj.8.2.7907072. FASEB J. 1994. PMID: 7907072 Review.
-
Defective function of GABA-containing synaptic vesicles in mice lacking the AP-3B clathrin adaptor.J Cell Biol. 2004 Oct 25;167(2):293-302. doi: 10.1083/jcb.200405032. Epub 2004 Oct 18. J Cell Biol. 2004. PMID: 15492041 Free PMC article.
-
Biogenesis of synaptic-like microvesicles in perforated PC12 cells.Methods. 1998 Oct;16(2):160-9. doi: 10.1006/meth.1998.0663. Methods. 1998. PMID: 9790862
-
A gamma-aminobutyric acid transporter driven by a proton pump is present in synaptic-like microvesicles of pancreatic beta cells.Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5317-21. doi: 10.1073/pnas.90.11.5317. Proc Natl Acad Sci U S A. 1993. PMID: 8506380 Free PMC article.
-
Synaptic vesicle biogenesis.Annu Rev Cell Dev Biol. 1999;15:733-98. doi: 10.1146/annurev.cellbio.15.1.733. Annu Rev Cell Dev Biol. 1999. PMID: 10611977 Review.
Cited by
-
Transcellular neuroligin-2 interactions enhance insulin secretion and are integral to pancreatic β cell function.J Biol Chem. 2012 Jun 8;287(24):19816-26. doi: 10.1074/jbc.M111.280537. Epub 2012 Apr 23. J Biol Chem. 2012. PMID: 22528485 Free PMC article.
-
Widespread dysregulation of peptide hormone release in mice lacking adaptor protein AP-3.PLoS Genet. 2013;9(9):e1003812. doi: 10.1371/journal.pgen.1003812. Epub 2013 Sep 26. PLoS Genet. 2013. PMID: 24086151 Free PMC article.
-
Nutritional energy stimulates NAD+ production to promote tankyrase-mediated PARsylation in insulinoma cells.PLoS One. 2015 Apr 13;10(4):e0122948. doi: 10.1371/journal.pone.0122948. eCollection 2015. PLoS One. 2015. PMID: 25876076 Free PMC article.
-
The Amino Acid Transporters of the Glutamate/GABA-Glutamine Cycle and Their Impact on Insulin and Glucagon Secretion.Front Endocrinol (Lausanne). 2013 Dec 31;4:199. doi: 10.3389/fendo.2013.00199. Front Endocrinol (Lausanne). 2013. PMID: 24427154 Free PMC article. Review.
-
The effects of aging on the BTBR mouse model of autism spectrum disorder.Front Aging Neurosci. 2014 Sep 1;6:225. doi: 10.3389/fnagi.2014.00225. eCollection 2014. Front Aging Neurosci. 2014. PMID: 25225482 Free PMC article.
References
-
- Abderrahmani A, Niederhauser G, Plaisance V, Haefliger JA, Regazzi R, Waeber G. Neuronal traits are required for glucose-induced insulin secretion. FEBS Lett 565: 133–138, 2004 - PubMed
-
- Bonifacio E, Lernmark A, Dawkins RL. Serum exchange and use of dilutions have improved precision of measurement of islet cell antibodies. J Immunol Methods 106: 83–88, 1988 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

