R-spondin-1 is a novel beta-cell growth factor and insulin secretagogue
- PMID: 20442404
- PMCID: PMC2898385
- DOI: 10.1074/jbc.M110.129874
R-spondin-1 is a novel beta-cell growth factor and insulin secretagogue
Abstract
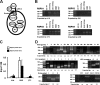
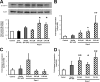
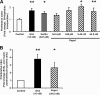
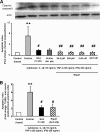
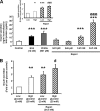
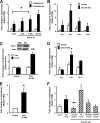
R-spondin-1 (Rspo1) is an intestinal growth factor known to exert its effects through activation of the canonical Wnt (cWnt) signaling pathway and subsequent expression of cWnt target genes. We have detected Rspo1 mRNA in murine islets and the murine MIN6 and betaTC beta-cell lines, and Rspo1 protein in MIN6 beta-cells. Rspo1 activated cWnt signaling in MIN6 beta-cells by increasing nuclear beta-catenin and c-myc, a cWnt target gene. Rspo1 also induced insulin mRNA expression in MIN6 cells. Analysis of MIN6 and mouse beta-cell proliferation by [(3)H]thymidine and BrdU incorporation, respectively, revealed that Rspo1 stimulated cell growth. Incubation of MIN6 and mouse beta-cells with cytokines (IL1beta/TNFalpha/interferon-gamma) significantly increased cellular apoptosis; this increase was abolished by pretreatment with Rspo1. Rspo1 also stimulated insulin secretion in a glucose-independent fashion. We further demonstrated that the glucagon-like peptide-1 receptor agonist, exendin4 (EX4), stimulated Rspo1 mRNA transcript levels in MIN6 cells in a glucose-, time-, dose-, and PI3-kinase-dependent fashion. This effect was not limited to this beta-cell line, as similar time-dependent increases in Rspo1 were also observed in the betaTC beta-cell line and mouse islets in response to EX4 treatment. Together, these studies demonstrate that Rspo1 is a novel beta-cell growth factor and insulin secretagogue that is regulated by EX4. These findings suggest that Rspo1 and the cWnt signaling pathway may serve as a novel target to enhance beta-cell growth and function in patients with type 2 diabetes.
Figures
References
-
- Butler A. E., Janson J., Bonner-Weir S., Ritzel R., Rizza R. A., Butler P. C. (2003) Diabetes 52, 102–110 - PubMed
-
- Welters H. J., Kulkarni R. N. (2008) Trends Endocrinol. Metab. 19, 349–355 - PubMed
-
- Nusse R. (2005) Cell Res. 15, 28–32 - PubMed
-
- Fujino T., Asaba H., Kang M. J., Ikeda Y., Sone H., Takada S., Kim D. H., Ioka R. X., Ono M., Tomoyori H., Okubo M., Murase T., Kamataki A., Yamamoto J., Magoori K., Takahashi S., Miyamoto Y., Oishi H., Nose M., Okazaki M., Usui S., Imaizumi K., Yanagisawa M., Sakai J., Yamamoto T. T. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 229–234 - PMC - PubMed
-
- Schinner S., Ulgen F., Papewalis C., Schott M., Woelk A., Vidal-Puig A., Scherbaum W. A. (2008) Diabetologia 51, 147–154 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

