Knock-in mutation reveals an essential role for focal adhesion kinase activity in blood vessel morphogenesis and cell motility-polarity but not cell proliferation
- PMID: 20442405
- PMCID: PMC2898428
- DOI: 10.1074/jbc.M110.129999
Knock-in mutation reveals an essential role for focal adhesion kinase activity in blood vessel morphogenesis and cell motility-polarity but not cell proliferation
Abstract
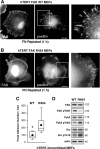
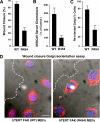
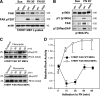
Focal adhesion kinase (FAK) associates with both integrins and growth factor receptors in the control of cell motility and survival. Loss of FAK during mouse development results in lethality at embryonic day 8.5 (E8.5) and a block in cell proliferation. Because FAK serves as both a scaffold and signaling protein, gene knock-outs do not provide mechanistic insights in distinguishing between these modes of FAK function. To determine the role of FAK activity during development, a knock-in point mutation (lysine 454 to arginine (R454)) within the catalytic domain was introduced by homologous recombination. Homozygous FAK(R454/R454) mutation was lethal at E9.5 with defects in blood vessel formation as determined by lack of yolk sac primary capillary plexus formation and disorganized endothelial cell patterning in FAK(R454/R454) embryos. In contrast to the inability of embryonic FAK(-/-) cells to proliferate ex vivo, primary FAK(R454/R454) mouse embryo fibroblasts (MEFs) were established from E8.5 embryos. R454 MEFs exhibited no difference in cell growth compared with normal MEFs, and R454 FAK localized to focal adhesions but was not phosphorylated at Tyr-397. In E8.5 embryos and primary MEFs, FAK R454 mutation resulted in decreased c-Src Tyr-416 phosphorylation. R454 MEFs exhibited enhanced focal adhesion formation, decreased migration, and defects in cell polarity. Within immortalized MEFs, FAK activity was required for fibronectin-stimulated FAK-p190RhoGAP association and p190RhoGAP tyrosine phosphorylation linked to decreased RhoA GTPase activity, focal adhesion turnover, and directional motility. Our results establish that intrinsic FAK activity is essential for developmental processes controlling blood vessel formation and cell motility-polarity but not cell proliferation. This work supports the use of FAK inhibitors to disrupt neovascularization.
Figures





References
-
- Serini G., Valdembri D., Bussolino F. (2006) Exp. Cell Res. 312, 651–658 - PubMed
-
- Jain R. K. (2003) Nat. Med. 9, 685–693 - PubMed
-
- Risau W. (1997) Nature 386, 671–674 - PubMed
-
- Hynes R. O. (2007) J. Thromb. Haemost. 5, Suppl. 1, 32–40 - PubMed
-
- Giancotti F. G., Ruoslahti E. (1999) Science 285, 1028–1032 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

