Protein-arginine methyltransferase 1 suppresses megakaryocytic differentiation via modulation of the p38 MAPK pathway in K562 cells
- PMID: 20442406
- PMCID: PMC2898291
- DOI: 10.1074/jbc.M109.092411
Protein-arginine methyltransferase 1 suppresses megakaryocytic differentiation via modulation of the p38 MAPK pathway in K562 cells
Abstract
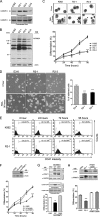
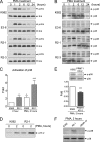
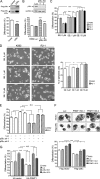
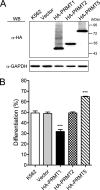
Protein-arginine methyltransferase 1 (PRMT1) plays pivotal roles in various cellular processes. However, its role in megakaryocytic differentiation has yet to be investigated. Human leukemia K562 cells have been used as a model to study hematopoietic differentiation. In this study, we report that ectopic expression of HA-PRMT1 in K562 cells suppressed phorbol 12-myristate 13-acetate (PMA)-induced megakaryocytic differentiation as demonstrated by changes in cytological characteristics, adhesive properties, and CD41 expression, whereas knockdown of PRMT1 by small interference RNA promoted differentiation. Impairment of the methyltransferase activity of PRMT1 diminished the suppressive effect. These results provide evidence for a novel role of PRMT1 in negative regulation of megakaryocytic differentiation. Activation of ERK MAPK has been shown to be essential for megakaryocytic differentiation, although the role of p38 MAPK is still poorly understood. We show that knockdown of p38alpha MAPK or treatment with the p38 inhibitor SB203580 significantly enhanced PMA-induced megakaryocytic differentiation. Further investigation revealed that PRMT1 promotes activation of p38 MAPK without inhibiting activation of ERK MAPK. In p38alpha knockdown cells, PRMT1 could no longer suppress differentiation. In contrast, enforced expression of p38alpha MAPK suppressed PMA-induced megakaryocytic differentiation of parental K562 as well as PRMT1-knockdown cells. We propose modulation of the p38 MAPK pathway by PRMT1 as a novel mechanism regulating megakaryocytic differentiation. This study thus provides a new perspective on the promotion of megakaryopoiesis.
Figures



Similar articles
-
Protein arginine methyltransferase 1 interacts with and activates p38α to facilitate erythroid differentiation.PLoS One. 2013;8(3):e56715. doi: 10.1371/journal.pone.0056715. Epub 2013 Mar 6. PLoS One. 2013. PMID: 23483889 Free PMC article.
-
The MKK-Dependent Phosphorylation of p38α Is Augmented by Arginine Methylation on Arg49/Arg149 during Erythroid Differentiation.Int J Mol Sci. 2020 May 17;21(10):3546. doi: 10.3390/ijms21103546. Int J Mol Sci. 2020. PMID: 32429593 Free PMC article.
-
Extracellular signal-regulated kinase/90-KDA ribosomal S6 kinase/nuclear factor-kappa B pathway mediates phorbol 12-myristate 13-acetate-induced megakaryocytic differentiation of K562 cells.J Biol Chem. 2001 Apr 20;276(16):13186-91. doi: 10.1074/jbc.M008092200. Epub 2001 Jan 29. J Biol Chem. 2001. PMID: 11278385
-
Roles of protein arginine methyltransferase 1 (PRMT1) in brain development and disease.Biochim Biophys Acta Gen Subj. 2021 Jan;1865(1):129776. doi: 10.1016/j.bbagen.2020.129776. Epub 2020 Oct 28. Biochim Biophys Acta Gen Subj. 2021. PMID: 33127433 Review.
-
The role of PRMT1 in cellular regulation and disease: Insights into biochemical functions and emerging inhibitors for cancer therapy.Eur J Pharm Sci. 2025 Jan 1;204:106958. doi: 10.1016/j.ejps.2024.106958. Epub 2024 Nov 7. Eur J Pharm Sci. 2025. PMID: 39521191 Review.
Cited by
-
Protein Arginine Methyltransferases (PRMTs): promising targets for the treatment of pulmonary disorders.Int J Mol Sci. 2012 Sep 27;13(10):12383-400. doi: 10.3390/ijms131012383. Int J Mol Sci. 2012. PMID: 23202904 Free PMC article. Review.
-
The extracellular signal-regulated kinase 1/2 modulates the intracellular localization of DNA methyltransferase 3A to regulate erythrocytic differentiation.Am J Transl Res. 2020 Mar 15;12(3):1016-1030. eCollection 2020. Am J Transl Res. 2020. PMID: 32269731 Free PMC article.
-
Use of zinc-finger nucleases to knock out the WAS gene in K562 cells: a human cellular model for Wiskott-Aldrich syndrome.Dis Model Mech. 2013 Mar;6(2):544-54. doi: 10.1242/dmm.010652. Epub 2013 Jan 11. Dis Model Mech. 2013. PMID: 23324327 Free PMC article.
-
Protein arginine methyltransferase 1 is required for maintenance of normal adult hematopoiesis.Int J Biol Sci. 2019 Oct 23;15(13):2763-2773. doi: 10.7150/ijbs.38859. eCollection 2019. Int J Biol Sci. 2019. PMID: 31853216 Free PMC article.
-
FAM98A promotes proliferation of non-small cell lung cancer cells via the P38-ATF2 signaling pathway.Cancer Manag Res. 2018 Jul 27;10:2269-2278. doi: 10.2147/CMAR.S163323. eCollection 2018. Cancer Manag Res. 2018. PMID: 30100758 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

