FimH can directly activate human and murine natural killer cells via TLR4
- PMID: 20442710
- PMCID: PMC2911256
- DOI: 10.1038/mt.2010.75
FimH can directly activate human and murine natural killer cells via TLR4
Abstract
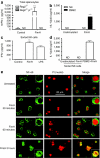
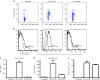
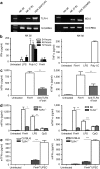
Although the importance of natural killer (NK) cells in innate immune responses against tumors or viral infections are well documented, their ability to directly recognize pathogens is less well defined. We have recently reported FimH, a bacterial fimbrial protein, as a novel Toll-like receptor (TLR)4 ligand that potently induces antiviral responses. Here, we investigated whether FimH either directly or indirectly can activate human and murine NK cells. We demonstrate that FimH potently activates both human and murine NK cells in vitro to induce cytokines [interferon (IFN)-gamma and tumor necrosis factor (TNF)-alpha] and cytotoxicity. Importantly, NK cells directly recognize FimH-expressing pathogens as FimH(+), but not FimH(-), bacteria were able to activate human NK cells. FimH activation of NK cells required TLR4 and MyD88 signaling, as NK cells from both TLR4(-/-) and MyD88(-/-) mice as well as human NK-92 cells, which lack TLR4, were all unresponsive to FimH. In addition, TLR4 neutralization significantly abrogated the response of human NK cells to FimH. Activation of purified NK cells by FimH was independent of lipopolysaccharide (LPS) or other bacterial contaminations. These data demonstrate for the first time that highly purified NK cells directly recognize and respond to FimH via TLR4-MyD88 pathways to aid innate protection against cancer or microbial infections.
Figures
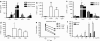

Similar articles
-
FimH adhesin of type 1 fimbriae is a potent inducer of innate antimicrobial responses which requires TLR4 and type 1 interferon signalling.PLoS Pathog. 2008 Dec;4(12):e1000233. doi: 10.1371/journal.ppat.1000233. Epub 2008 Dec 5. PLoS Pathog. 2008. PMID: 19057665 Free PMC article.
-
Cutting edge: FimH adhesin of type 1 fimbriae is a novel TLR4 ligand.J Immunol. 2008 Nov 15;181(10):6702-6. doi: 10.4049/jimmunol.181.10.6702. J Immunol. 2008. PMID: 18981086
-
FimH, a TLR4 ligand, induces innate antiviral responses in the lung leading to protection against lethal influenza infection in mice.Antiviral Res. 2011 Nov;92(2):346-55. doi: 10.1016/j.antiviral.2011.09.004. Epub 2011 Sep 14. Antiviral Res. 2011. PMID: 21945041
-
FimH as a mucosal adjuvant enhances persistent antibody response and protective efficacy of the anti-caries vaccine.Arch Oral Biol. 2019 May;101:122-129. doi: 10.1016/j.archoralbio.2019.02.009. Epub 2019 Feb 18. Arch Oral Biol. 2019. PMID: 30927661
-
FAM26F: An Enigmatic Protein Having a Complex Role in the Immune System.Int Rev Immunol. 2023;42(4):247-257. doi: 10.1080/08830185.2016.1206098. Epub 2016 Sep 19. Int Rev Immunol. 2023. PMID: 27645024 Review.
Cited by
-
Noninvasive Identification of Immune-Related Biomarkers in Hepatocellular Carcinoma.J Oncol. 2019 Aug 18;2019:2531932. doi: 10.1155/2019/2531932. eCollection 2019. J Oncol. 2019. PMID: 31531018 Free PMC article.
-
Natural killer cells take two tolls to destruct bile ducts.Hepatology. 2011 Apr;53(4):1076-9. doi: 10.1002/hep.24275. Hepatology. 2011. PMID: 21384404 Free PMC article. No abstract available.
-
Natural killer cell-mediated host defense against uropathogenic E. coli is counteracted by bacterial hemolysinA-dependent killing of NK cells.Cell Host Microbe. 2013 Dec 11;14(6):664-74. doi: 10.1016/j.chom.2013.11.004. Cell Host Microbe. 2013. PMID: 24331464 Free PMC article.
-
Cloning and Expression of Pigeon-Derived Escherichia coli Type 1 Pilus Clusters and Analysis of Amino Acid Sequence Characteristics of Functional Proteins.Genes (Basel). 2024 Sep 26;15(10):1253. doi: 10.3390/genes15101253. Genes (Basel). 2024. PMID: 39457377 Free PMC article.
-
A Dynamic Interplay of Innate Immune Responses During Urinary Tract Infection.Immune Netw. 2024 Jul 22;24(4):e31. doi: 10.4110/in.2024.24.e31. eCollection 2024 Aug. Immune Netw. 2024. PMID: 39246616 Free PMC article. Review.
References
-
- Biron CA, Nguyen KB, Pien GC, Cousens LP., and, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. - PubMed
-
- Wu J., and, Lanier LL. Natural killer cells and cancer. Adv Cancer Res. 2003;90:127–156. - PubMed
-
- Chalifour A, Jeannin P, Gauchat JF, Blaecke A, Malissard M, N'Guyen T, et al. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers α-defensin production. Blood. 2004;104:1778–1783. - PubMed
-
- Raulet DH. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat Immunol. 2004;5:996–1002. - PubMed
-
- Biron CA., and, Brossay L. NK cells and NKT cells in innate defense against viral infections. Curr Opin Immunol. 2001;13:458–464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

