Sec24D-dependent transport of extracellular matrix proteins is required for zebrafish skeletal morphogenesis
- PMID: 20442775
- PMCID: PMC2860987
- DOI: 10.1371/journal.pone.0010367
Sec24D-dependent transport of extracellular matrix proteins is required for zebrafish skeletal morphogenesis
Abstract
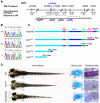
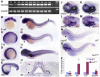
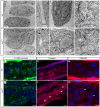
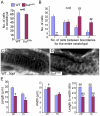
Protein transport from endoplasmic reticulum (ER) to Golgi is primarily conducted by coated vesicular carriers such as COPII. Here, we describe zebrafish bulldog mutations that disrupt the function of the cargo adaptor Sec24D, an integral component of the COPII complex. We show that Sec24D is essential for secretion of cartilage matrix proteins, whereas the preceding development of craniofacial primordia and pre-chondrogenic condensations does not depend on this isoform. Bulldog chondrocytes fail to secrete type II collagen and matrilin to extracellular matrix (ECM), but membrane bound receptor beta1-Integrin and Cadherins appear to leave ER in Sec24D-independent fashion. Consequently, Sec24D-deficient cells accumulate proteins in the distended ER, although a subset of ER compartments and Golgi complexes as visualized by electron microscopy and NBD C(6)-ceramide staining appear functional. Consistent with the backlog of proteins in the ER, chondrocytes activate the ER stress response machinery and significantly upregulate BiP transcription. Failure of ECM secretion hinders chondroblast intercalations thus resulting in small and malformed cartilages and severe craniofacial dysmorphology. This defect is specific to Sec24D mutants since knockdown of Sec24C, a close paralog of Sec24D, does not result in craniofacial cartilage dysmorphology. However, craniofacial development in double Sec24C/Sec24D-deficient animals is arrested earlier than in bulldog/sec24d, suggesting that Sec24C can compensate for loss of Sec24D at initial stages of chondrogenesis, but Sec24D is indispensable for chondrocyte maturation. Our study presents the first developmental perspective on Sec24D function and establishes Sec24D as a strong candidate for cartilage maintenance diseases and craniofacial birth defects.
Conflict of interest statement
Figures


Similar articles
-
The feelgood mutation in zebrafish dysregulates COPII-dependent secretion of select extracellular matrix proteins in skeletal morphogenesis.Dis Model Mech. 2011 Nov;4(6):763-76. doi: 10.1242/dmm.007625. Epub 2011 Jul 4. Dis Model Mech. 2011. PMID: 21729877 Free PMC article.
-
Secretory COPII coat component Sec23a is essential for craniofacial chondrocyte maturation.Nat Genet. 2006 Oct;38(10):1198-203. doi: 10.1038/ng1880. Epub 2006 Sep 17. Nat Genet. 2006. PMID: 16980978
-
Collagen has a unique SEC24 preference for efficient export from the endoplasmic reticulum.Traffic. 2022 Jan;23(1):81-93. doi: 10.1111/tra.12826. Epub 2021 Nov 22. Traffic. 2022. PMID: 34761479 Free PMC article.
-
Traffic jams in fish bones: ER-to-Golgi protein transport during zebrafish development.Cell Adh Migr. 2011 Mar-Apr;5(2):114-8. doi: 10.4161/cam.5.2.14377. Epub 2011 Mar 1. Cell Adh Migr. 2011. PMID: 21178403 Free PMC article. Review.
-
ER-to-Golgi transport: form and formation of vesicular and tubular carriers.Biochim Biophys Acta. 2005 Jul 10;1744(3):304-15. doi: 10.1016/j.bbamcr.2005.03.003. Epub 2005 Mar 23. Biochim Biophys Acta. 2005. PMID: 15979504 Review.
Cited by
-
Proteomic and functional analyses reveal MAPK1 regulates milk protein synthesis.Molecules. 2012 Dec 27;18(1):263-75. doi: 10.3390/molecules18010263. Molecules. 2012. PMID: 23271465 Free PMC article.
-
An Update on Animal Models of Osteogenesis Imperfecta.Calcif Tissue Int. 2022 Oct;111(4):345-366. doi: 10.1007/s00223-022-00998-6. Epub 2022 Jun 29. Calcif Tissue Int. 2022. PMID: 35767009 Review.
-
The feelgood mutation in zebrafish dysregulates COPII-dependent secretion of select extracellular matrix proteins in skeletal morphogenesis.Dis Model Mech. 2011 Nov;4(6):763-76. doi: 10.1242/dmm.007625. Epub 2011 Jul 4. Dis Model Mech. 2011. PMID: 21729877 Free PMC article.
-
Zebrafish Models for Skeletal and Extraskeletal Osteogenesis Imperfecta Features: Unveiling Pathophysiology and Paving the Way for Drug Discovery.Calcif Tissue Int. 2024 Dec;115(6):931-959. doi: 10.1007/s00223-024-01282-5. Epub 2024 Sep 25. Calcif Tissue Int. 2024. PMID: 39320469 Free PMC article. Review.
-
[Research progress on collagen secretion mechanisms in scarring].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2025 Mar 25;54(2):266-278. doi: 10.3724/zdxbyxb-2024-0535. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2025. PMID: 40194913 Free PMC article. Review. Chinese.
References
-
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, et al. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. - PubMed
-
- Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. - PubMed
-
- Fromme JC, Orci L, Schekman R. Coordination of COPII vesicle trafficking by Sec23. Trends Cell Biol. 2008;18:330–336. - PubMed
-
- Boyadjiev SA, Fromme JC, Ben J, Chong SS, Nauta C, et al. Cranio-lenticulo-sutural dysplasia is caused by a SEC23A mutation leading to abnormal endoplasmic-reticulum-to-Golgi trafficking. Nat Genet. 2006;38:1192–1197. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

