Doxorubicin in combination with a small TGFbeta inhibitor: a potential novel therapy for metastatic breast cancer in mouse models
- PMID: 20442777
- PMCID: PMC2860989
- DOI: 10.1371/journal.pone.0010365
Doxorubicin in combination with a small TGFbeta inhibitor: a potential novel therapy for metastatic breast cancer in mouse models
Abstract
Background: Recent studies suggested that induction of epithelial-mesenchymal transition (EMT) might confer both metastatic and self-renewal properties to breast tumor cells resulting in drug resistance and tumor recurrence. TGFbeta is a potent inducer of EMT and has been shown to promote tumor progression in various breast cancer cell and animal models.
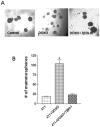
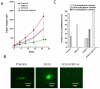
Principal findings: We report that chemotherapeutic drug doxorubicin activates TGFbeta signaling in human and murine breast cancer cells. Doxorubicin induced EMT, promoted invasion and enhanced generation of cells with stem cell phenotype in murine 4T1 breast cancer cells in vitro, which were significantly inhibited by a TGFbeta type I receptor kinase inhibitor (TbetaRI-KI). We investigated the potential synergistic anti-tumor activity of TbetaR1-KI in combination with doxorubicin in animal models of metastatic breast cancer. Combination of Doxorubicin and TbetaRI-KI enhanced the efficacy of doxorubicin in reducing tumor growth and lung metastasis in the 4T1 orthotopic xenograft model in comparison to single treatments. Doxorubicin treatment alone enhanced metastasis to lung in the human breast cancer MDA-MB-231 orthotopic xenograft model and metastasis to bone in the 4T1 orthotopic xenograft model, which was significantly blocked when TbetaR1-KI was administered in combination with doxorubicin.
Conclusions: These observations suggest that the adverse activation of TGFbeta pathway by chemotherapeutics in the cancer cells together with elevated TGFbeta levels in tumor microenvironment may lead to EMT and generation of cancer stem cells resulting in the resistance to the chemotherapy. Our results indicate that the combination treatment of doxorubicin with a TGFbeta inhibitor has the potential to reduce the dose and consequently the toxic side-effects of doxorubicin, and improve its efficacy in the inhibition of breast cancer growth and metastasis.
Conflict of interest statement
Figures
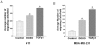


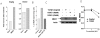

References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Sledge GW, Neuberg D, Bernardo P, Ingle JN, Martino S, et al. Phase III trial of doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as front-line chemotherapy for metastatic breast cancer: an intergroup trial (E1193). J Clin Oncol. 2003;21:588–592. - PubMed
-
- Ellis MJ, Hayes DF, Lippman ML. Treatment of metastatic breast cancer: Diseases of the breast. 2004;3rd:749–797.
-
- Chen JH, Ling R, Yao Q, Li Y, Chen T, et al. Effect of small-sized liposomal Adriamycin administered by various routes on a metastatic breast cancer model. Endocr Relat Cancer. 2005;12:93–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

