Polyglutamine induced misfolding of huntingtin exon1 is modulated by the flanking sequences
- PMID: 20442863
- PMCID: PMC2861695
- DOI: 10.1371/journal.pcbi.1000772
Polyglutamine induced misfolding of huntingtin exon1 is modulated by the flanking sequences
Abstract
Polyglutamine (polyQ) expansion in exon1 (XN1) of the huntingtin protein is linked to Huntington's disease. When the number of glutamines exceeds a threshold of approximately 36-40 repeats, XN1 can readily form amyloid aggregates similar to those associated with disease. Many experiments suggest that misfolding of monomeric XN1 plays an important role in the length-dependent aggregation. Elucidating the misfolding of a XN1 monomer can help determine the molecular mechanism of XN1 aggregation and potentially help develop strategies to inhibit XN1 aggregation. The flanking sequences surrounding the polyQ region can play a critical role in determining the structural rearrangement and aggregation mechanism of XN1. Few experiments have studied XN1 in its entirety, with all flanking regions. To obtain structural insights into the misfolding of XN1 toward amyloid aggregation, we perform molecular dynamics simulations on monomeric XN1 with full flanking regions, a variant missing the polyproline regions, which are hypothesized to prevent aggregation, and an isolated polyQ peptide (Q(n)). For each of these three constructs, we study glutamine repeat lengths of 23, 36, 40 and 47. We find that polyQ peptides have a positive correlation between their probability to form a beta-rich misfolded state and their expansion length. We also find that the flanking regions of XN1 affect its probability to form a beta-rich state compared to the isolated polyQ. Particularly, the polyproline regions form polyproline type II helices and decrease the probability of the polyQ region to form a beta-rich state. Additionally, by lengthening polyQ, the first N-terminal 17 residues are more likely to adopt a beta-sheet conformation rather than an alpha-helix conformation. Therefore, our molecular dynamics study provides a structural insight of XN1 misfolding and elucidates the possible role of the flanking sequences in XN1 aggregation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
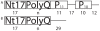

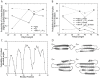

References
-
- Truant R, Atwal RS, Desmond C, Munsie L, Tran T. Huntington's disease: revisiting the aggregation hypothesis in polyglutamine neurodegenerative diseases. FEBS J. 2008;275:4252–62. - PubMed
-
- Ross CA. Polyglutamine Pathogenesis: Emergence of Unifying Mechanisms for Huntington's Disease and Related Disorders. Neuron. 2002;35:819–822. - PubMed
-
- Saunders HM, Bottomley SP. Multi-domain misfolding: understanding the aggregation pathway of polyglutamine proteins. Protein Eng Des Sel. 2009;22:447–51. - PubMed
-
- Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10(Suppl):S10–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

