Complement as an endogenous adjuvant for dendritic cell-mediated induction of retrovirus-specific CTLs
- PMID: 20442876
- PMCID: PMC2861708
- DOI: 10.1371/journal.ppat.1000891
Complement as an endogenous adjuvant for dendritic cell-mediated induction of retrovirus-specific CTLs
Abstract
Previous studies have demonstrated the involvement of complement (C) in induction of efficient CTL responses against different viral infections, but the exact role of complement in this process has not been determined. We now show that C opsonization of retroviral particles enhances the ability of dendritic cells (DCs) to induce CTL responses both in vitro and in vivo. DCs exposed to C-opsonized HIV in vitro were able to stimulate CTLs to elicit antiviral activity significantly better than non-opsonized HIV. Furthermore, experiments using the Friend virus (FV) mouse model illustrated that the enhancing role of complement on DC-mediated CTL induction also occurred in vivo. Our results indicate that complement serves as natural adjuvant for DC-induced expansion and differentiation of specific CTLs against retroviruses.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

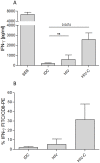

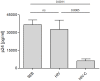
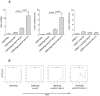
References
-
- Pantaleo G, Demarest JF, Soudeyns H, Graziosi C, Denis F, et al. Major expansion of CD8+ T cells with a predominant V beta usage during the primary immune response to HIV. Nature. 1994;370:463–467. - PubMed
-
- McMichael AJ, Rowland-Jones SL. Cellular immune responses to HIV. Nature. 2001;410:980–987. - PubMed
-
- Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27:406–416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

