Total synthesis and structural revision of vannusals A and B: synthesis of the originally assigned structure of vannusal B
- PMID: 20443561
- PMCID: PMC2881636
- DOI: 10.1021/ja100740t
Total synthesis and structural revision of vannusals A and B: synthesis of the originally assigned structure of vannusal B
Abstract
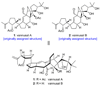
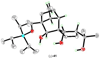
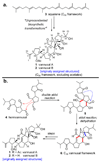
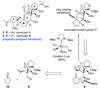
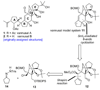
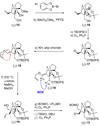
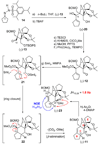
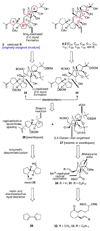
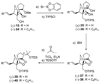
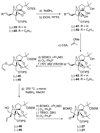
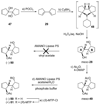
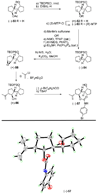
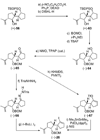
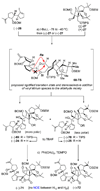
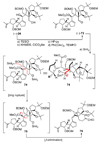
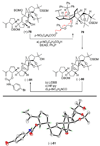
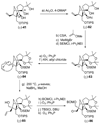
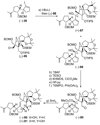
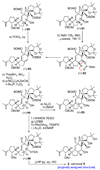
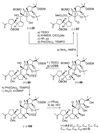
The total synthesis of the originally assigned structure of vannusal B (2) and its diastereomer (d-2) are described. Initial forays into these structures with model systems revealed the viability of a metathesis-based approach and a SmI(2)-mediated strategy for the key cyclization to forge the central region of the molecule, ring C. The former approach was abandoned in favor of the latter when more functionalized substrates failed to enter the cyclization process. The successful, devised convergent strategy based on the SmI(2)-mediated ring closure utilized vinyl iodide (-)-26 and aldehyde fragment (+/-)-86 as key building blocks, whose lithium-mediated coupling led to isomeric coupling products (+)-87 and (-)-88 (as shown in Scheme 17 in the article). Intermediate (-)-88 was converted, via (-)-89 and (-)-90/(+)-91, to vannusal B structure 2 (as shown in Scheme 18 in the article), whose spectroscopic data did not match those reported for the natural product. Similarly, intermediate (+)-25, obtained through coupling of vinyl iodide (-)-26 and aldehyde (+/-)-27 (as shown in Scheme 13 in the article) was transformed via intermediates (-)-97 and (+)-98 (as shown in Scheme 19 in the article) to diastereomeric vannusal B structure (+)-d-2 (as shown in Scheme 19 in the article) which was also proven spectroscopically to be non-identical to the naturally occurring substance. These investigations led to the discovery and development of a number of new synthetic technologies that set the stage for the solution of the vannusal structural conundrum.
Figures



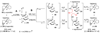
Similar articles
-
Total synthesis and structural revision of vannusals A and B: synthesis of the true structures of vannusals A and B.J Am Chem Soc. 2010 May 26;132(20):7153-76. doi: 10.1021/ja100742b. J Am Chem Soc. 2010. PMID: 20443558 Free PMC article.
-
Total synthesis of the originally assigned structure of vannusal B.Angew Chem Int Ed Engl. 2008;47(45):8605-10. doi: 10.1002/anie.200804228. Angew Chem Int Ed Engl. 2008. PMID: 18850598 Free PMC article. No abstract available.
-
Samarium(II)-mediated spirocyclization by intramolecular aryl radical addition onto an aromatic ring.J Org Chem. 2008 Sep 19;73(18):7145-52. doi: 10.1021/jo800656a. Epub 2008 Aug 13. J Org Chem. 2008. PMID: 18698825
-
Synthesis of Nitrogen Heterocycles Using Samarium(II) Iodide.Molecules. 2017 Nov 21;22(11):2018. doi: 10.3390/molecules22112018. Molecules. 2017. PMID: 29160806 Free PMC article. Review.
-
SmI(2)-induced reductive cyclizations for the synthesis of cyclic ethers and applications in natural product synthesis.Chem Soc Rev. 2010 Jun;39(6):1955-72. doi: 10.1039/b902737h. Epub 2010 Mar 23. Chem Soc Rev. 2010. PMID: 20502797 Review.
Cited by
-
Synthetic Strategies toward Natural Products Containing Contiguous Stereogenic Quaternary Carbon Atoms.Angew Chem Int Ed Engl. 2016 Mar 18;55(13):4156-86. doi: 10.1002/anie.201507549. Epub 2016 Feb 2. Angew Chem Int Ed Engl. 2016. PMID: 26836448 Free PMC article. Review.
-
Recent advances of N-heterocyclic carbenes in the applications of constructing carbo- and heterocyclic frameworks with potential biological activity.RSC Adv. 2021 Nov 26;11(60):38060-38078. doi: 10.1039/d1ra06155k. eCollection 2021 Nov 23. RSC Adv. 2021. PMID: 35498096 Free PMC article. Review.
-
Marine Natural Products from Indonesian Waters.Mar Drugs. 2019 Jun 19;17(6):364. doi: 10.3390/md17060364. Mar Drugs. 2019. PMID: 31248122 Free PMC article. Review.
-
Highly Accurate Prediction of NMR Chemical Shifts from Low-Level Quantum Mechanics Calculations Using Machine Learning.J Chem Theory Comput. 2024 Mar 12;20(5):2152-2166. doi: 10.1021/acs.jctc.3c01256. Epub 2024 Feb 8. J Chem Theory Comput. 2024. PMID: 38331423 Free PMC article.
-
Survey of marine natural product structure revisions: a synergy of spectroscopy and chemical synthesis.Bioorg Med Chem. 2011 Nov 15;19(22):6675-701. doi: 10.1016/j.bmc.2011.06.011. Epub 2011 Jun 12. Bioorg Med Chem. 2011. PMID: 21715178 Free PMC article. Review.
References
-
- Guella G, Dini F, Pietra F. Angew. Chem., Int. Ed. 1999;38:1134–1136. - PubMed
-
- Guella G, Callone E, Di Giuseppe G, Frassanito R, Frontini FP, Mancini I, Dini F. Eur. J. Org. Chem. 2007:5226–5234.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

