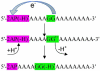
Proton-coupled electron transfer in DNA on formation of radiation-produced ion radicals
- PMID: 20443634
- PMCID: PMC2947616
- DOI: 10.1021/cr100023g
Proton-coupled electron transfer in DNA on formation of radiation-produced ion radicals
Figures

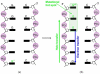
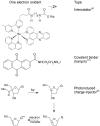
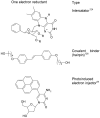


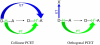
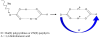


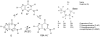
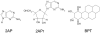

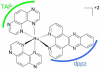
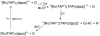
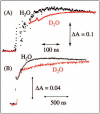
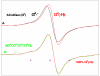





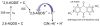

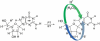


References
-
- Russell P. iGenetics. Benjamin Cummings; New York: 2001.
-
- Saenger W. Principles of Nucleic Acid Structure. Springer-Verlag; New York: 1984.
-
- von Sonntag C. The chemical basis of radiation biology. Taylor and Francis; London, New York, Philadelphia: 1987.
-
- Swiderek P. Angew. Chem. Int. Ed. 2006;45:4056. and references therein. - PubMed
-
- Turecek F. Adv. Quantum. Chem. 2007;52:89.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

