BMP-2 and FGF-2 synergistically facilitate adoption of a cardiac phenotype in somatic bone marrow c-kit+/Sca-1+ stem cells
- PMID: 20443832
- PMCID: PMC2863128
- DOI: 10.1111/j.1752-8062.2008.00034.x
BMP-2 and FGF-2 synergistically facilitate adoption of a cardiac phenotype in somatic bone marrow c-kit+/Sca-1+ stem cells
Abstract
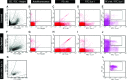
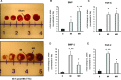
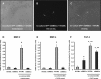
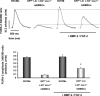
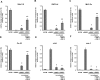
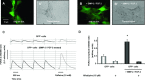
The aim of this study was to explore the effect of bone morphogenetic protein-2 (BMP-2) and fibroblast growth factor-2 (FGF-2)- paracrine factors implicated in both cardiac embryogenesis and cardiac repair following myocardial infarction (MI)-on murine bone marrow stem cell (mBMSC) differentiation in an ex vivo cardiac microenvironment. For this purpose, green fluorescent protein (GFP) expressing hematopoietic lineage negative (lin-) c-kit ligand (c-kit) and stem cell antigen-1 (Sca-1) positive (GFP-lin-/c-kit+/sca+) mBMSC were co-cultured with neonatal rat ventricular cardiomyocytes (NVCMs). GFP+ mBMSC significantly induced the expression of BMP-2 and FGF-2 in NVCMs, and approximately 4% GFP+ mBMSCs could be recovered from the co-culture at day 10. The addition of BMP-2 in concert with FGF-2 significantly enhanced the amount of integrated GFP+ mBMSCs by 5-fold ( approximately 20%), whereas the addition of anti-BMP-2 and/or anti-FGF-2 antibodies completely abolished this effect. An analysis of calcium cycling revealed robust calcium transients in GFP+ mBMSCs treated with BMP-2/FGF-2 compared to untreated co-cultures. BMP-2 and FGF-2 addition led to a significant induction of early (NK2 transcription factor related, locus 5; Nkx2.5, GATA binding protein 4; GATA-4) and late (myosin light chain kinase [MLC-2v], connexin 43 [Cx43]) cardiac marker mRNA expression in mBMSCs following co-culture. In addition, re-cultured fluorescence-activated cell sorting (FACS)-purified BMP-2/FGF-2-treated mBMSCs revealed robust calcium transients in response to electrical field stimulation which were inhibited by the L-type calcium channel (LTCC) inhibitor, nifedipine, and displayed caffeine-sensitive intracellular calcium stores. In summary, our results show that mBMSCs can adopt a functional cardiac phenotype through treatment with factors essential to embryonic cardiogenesis that are induced after cardiac ischemia. This study provides the first evidence that mBMSCs with long-term self-renewal potential possess the capability to serve as a functional cardiomyocyte precursor through the appropriate paracrine input and cross-talk within an appropriate cardiac microenvironment.
Keywords: BMP-2; FGF-2; mBMSCs; paracrine signalling.
Figures

Similar articles
-
Fibroblast growth factor-2 and -4 promote the proliferation of bone marrow mesenchymal stem cells by the activation of the PI3K-Akt and ERK1/2 signaling pathways.Stem Cells Dev. 2008 Aug;17(4):725-36. doi: 10.1089/scd.2007.0230. Stem Cells Dev. 2008. PMID: 18788932
-
Cardiomyocyte-like cell differentiation by FGF-2 transfection and induction of rat bone marrow mesenchymal stem cells.Tissue Cell. 2021 Dec;73:101665. doi: 10.1016/j.tice.2021.101665. Epub 2021 Oct 14. Tissue Cell. 2021. PMID: 34695652
-
Electrophysiological properties of mouse bone marrow c-kit+ cells co-cultured onto neonatal cardiac myocytes.Cardiovasc Res. 2005 Jun 1;66(3):482-92. doi: 10.1016/j.cardiores.2005.01.018. Cardiovasc Res. 2005. PMID: 15914113
-
Requirement for BMP and FGF signaling during cardiogenic induction in non-precardiac mesoderm is specific, transient, and cooperative.Dev Dyn. 2000 Jun;218(2):383-93. doi: 10.1002/(SICI)1097-0177(200006)218:2<383::AID-DVDY11>3.0.CO;2-P. Dev Dyn. 2000. PMID: 10842364
-
The roles and controls of GATA factors in blood and cardiac development.IUBMB Life. 2020 Jan;72(1):39-44. doi: 10.1002/iub.2178. Epub 2019 Nov 28. IUBMB Life. 2020. PMID: 31778014 Free PMC article. Review.
Cited by
-
Bioprintability: Physiomechanical and Biological Requirements of Materials for 3D Bioprinting Processes.Polymers (Basel). 2020 Oct 1;12(10):2262. doi: 10.3390/polym12102262. Polymers (Basel). 2020. PMID: 33019639 Free PMC article. Review.
-
Bone morphogenetic protein-2/-4 upregulation promoted by endothelial cells in coculture enhances mouse embryoid body differentiation.Stem Cells Dev. 2013 Dec 15;22(24):3252-60. doi: 10.1089/scd.2013.0013. Epub 2013 Sep 24. Stem Cells Dev. 2013. PMID: 23924071 Free PMC article.
-
Coculture with hematopoietic stem cells protects cardiomyocytes against apoptosis via paracrine activation of AKT.J Transl Med. 2012 Jun 6;10:115. doi: 10.1186/1479-5876-10-115. J Transl Med. 2012. PMID: 22672705 Free PMC article.
-
Cardiac regeneration: different cells same goal.Med Biol Eng Comput. 2011 Jul;49(7):723-32. doi: 10.1007/s11517-011-0776-5. Epub 2011 Apr 16. Med Biol Eng Comput. 2011. PMID: 21499802 Free PMC article. Review.
-
S100A1 in cardiovascular health and disease: closing the gap between basic science and clinical therapy.J Mol Cell Cardiol. 2009 Oct;47(4):445-55. doi: 10.1016/j.yjmcc.2009.06.003. Epub 2009 Jun 16. J Mol Cell Cardiol. 2009. PMID: 19538970 Free PMC article. Review.
References
-
- Chen SL, Fang W W, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S, Sun JP. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004; 94: 92–95. - PubMed
-
- Schachinger V, Assmus B, Britten MB, Honold J, Lehmann R, Teupe C, Abolmaali ND, Vogl TJ, Hofmann WK, Martin H, Dimmeler S, Zeiher AM. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one‐year results of the TOPCARE‐AMI Trial. J Am Coll Cardiol. 2004; 44: 1690–1699. - PubMed
-
- Wollert KC, Meyer GP, Lotz J, Ringes‐Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone‐marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004; 364: 141–148. - PubMed
-
- Badorff C, Brandes RP, Popp R, Rupp S, Urbich C, Aicher A, Fleming I, Busse R, Zeiher AM, Dimmeler S. Transdifferentiation of blood‐derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 2003; 107: 1024–1032. - PubMed
-
- Xu W, Zhang X, Qian H, Zhu W, Sun X, Hu J, Zhou H, Chen Y. Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp Biol Med (Maywood). 2004; 229: 623–631. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

