c-Kit+ bone marrow stem cells differentiate into functional cardiac myocytes
- PMID: 20443864
- PMCID: PMC4122301
- DOI: 10.1111/j.1752-8062.2008.00089.x
c-Kit+ bone marrow stem cells differentiate into functional cardiac myocytes
Abstract
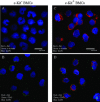
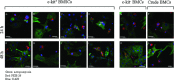
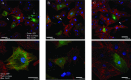
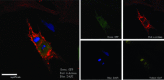
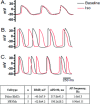
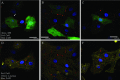
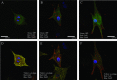
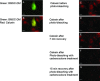
The utility of bone marrow cells (BMCs) to regenerate cardiac myocytes is controversial. The present study examined the capacity of different types of BMCs to generate functional cardiac myocytes. Isolated c-kit(+) BMCs (BMSCs), c-kit(+) and crude BMCs from the adult feline femur were membrane stained with PKH26 dye or infected with a control enhanced green fluorescence protein transcript (EGFP)-adenovirus prior to co-culture upon neonatal rat ventricular myocytes (NRVM). Co-cultured cells were immuno-stained for c-kit, alpha-tropomyosin, alpha-actinin, connexin 43 (Cx43) and Ki67 and analyzed with confocal microscopy. Electrophysiology of BMSC derived myocytes were compared to NRVMs within the same culture dish. Gap junction function was analyzed by fluorescence recovery after photo-bleaching (FRAP). BMCs proliferated and differentiated into cardiac myocytes during the first 48 hours of co-culturing. These newly formed cardiac myocytes were able to contract spontaneously or synchronously with neighboring NRVMs. The myogenic rate of c-kit(+) BMSCs was significantly greater than c-kit(+) and crude BMCs (41.2 +/- 2.1, 6.1 +/- 1.2, and 17.1 +/- 1.5%, respectively). The newly formed cardiac myocytes exhibited an immature electrophysiological phenotype until they became electrically coupled to NRVMs through functional gap junctions. BMSCs did not become functional myocytes in the absence of NRVMs. In conclusion, c-kit(+) BMSCs have the ability to transdifferentiate into functional cardiac myocytes.
Figures

References
-
- Kajstura J, Cheng W, Reiss K, Clark WA, Sonnenblick EH, Krajewski S, Reed JC, Olivetti G, Anversa P. Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Invest. 1996; 74(1): 86–107. - PubMed
-
- Shorofsky SR, Aggarwal R, Corretti M, Baffa JM, Strum JM, Al‐Seikhan BA, Kobayashi YM, Jones LR, Wier WG, Balke CW. Cellular mechanisms of altered contractility in the hypertrophied heart: big hearts, big sparks. Cir Res. 1999; 84(4): 424–434. - PubMed
-
- Bristow MR, Kantrowitz NE, Ginsburg R, Fowler MB. Beta‐adrenergic function in heart muscle disease and heart failure. J Mol Cell Cardiol. 1985; 17(Suppl 2): 41–52. - PubMed
-
- Ramasubbu K, Mann DL, Deswal A. Anti‐angiotensin therapy: new perspectives. Cardiol Clin. 2007; 25(4): 573–580; vi–vii. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

