Inhibition of Staphylococcus aureus biofilms by a novel antibacterial envelope for use with implantable cardiac devices
- PMID: 20443892
- PMCID: PMC5350741
- DOI: 10.1111/j.1752-8062.2009.00123.x
Inhibition of Staphylococcus aureus biofilms by a novel antibacterial envelope for use with implantable cardiac devices
Abstract
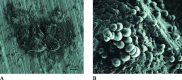
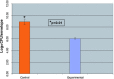
Biofilm formation on representative implantable medical devices using a known human pathogen (Staphylococcus aureus) was significantly reduced (p < 0.01) at all time points measured (24,48, and 72 hours) by employing a novel antibacterial envelope (AIGIS Rx). The result was demonstrated using a standard US Centers for Disease Control (CDC) bioreactor model and the results were confirmed by Scanning Electron Microscopy (SEM). The antibacterial envelope used in the study is coated with a proprietary combination broad spectrum antibiotics (rifampin and minocycline) embedded in a resorbable polymeric coating. The antibiotics are designed to elute out of the coating over a multi-day period for controlled, site-specific drug delivery. The infection rate for patients receiving pacemakers and defibrillators is increasing faster than the rate of new implants and the growing resistance of S. aureus strains suggests that conventional, systemic antibiotic prophylaxis may have limited future utility. Moreover, emerging evidence suggests that bacterial biofilms result in infections of implantable medical devices. These findings demonstrate the in vitro efficacy of a new means to address potential biofilm-derived Hospital Acquired Infections (HAIs) related to implantable medical devices composed of titanium inclusive of pacemakers and defibrillators by means of a locally delivered, low dose, combination antibacterial treatment.
Figures






References
-
- Wilkoff BL, Auricchio A, Brugada J, Cowie M, Ellenbogen KA, Gillis AM, Hayes DL, Hewlett JG, Kautzner J, Love A, Morgan JM, Priori SG, Reynolds DW, Schoenfeld MH, Vardas PE. HRS/EHRA expert consensus on the monitoring of cardiovascular implantable electronic devices (CIEDs) description of techniques, indications, personnel, frequency and ethical considerations. Heart Rhythm. 2008; 5(6): 907–925. - PubMed
-
- Chua JD, Wilkoff BL, Lee I, Juratli N, Longworth DL, Gordon SM. Diagnosis and management of infections involving implantable electrophysiologic cardiac devices. Ann Intern Med 2000; 133(8): 604–608. - PubMed
-
- Hedrick TL, Adams JD, Sawyer RG. Implant‐associated infections: an overview. J Long Term Eft Med Implants. 2006; 16(1): 83–99. - PubMed
-
- Klug D, Balde M, Pavin D, Hidden‐Lucet F, Clementy J, Sadoul N, Rey JL, Lande G, Lazarus A, Victor J, Barnay C, Grandbastien B, Kacet S. Risk factors related to infections of implanted pacemakers and cardioverter‐defibrillators: results of a large prospective study. Circulation. 2007; 116(12): 1349–1355. - PubMed
-
- Karchmer AW, Longworth DL. Infections of intracardiac devices. Infect Dis Clin North Am. 2002; 16(2): 477–505, xii. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

