Cathepsin L occupies a vacuolar compartment and is a protein maturase within the endo/exocytic system of Toxoplasma gondii
- PMID: 20444089
- PMCID: PMC2909120
- DOI: 10.1111/j.1365-2958.2010.07181.x
Cathepsin L occupies a vacuolar compartment and is a protein maturase within the endo/exocytic system of Toxoplasma gondii
Abstract
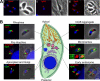
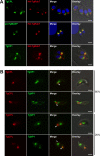
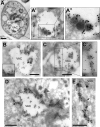
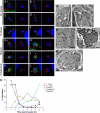
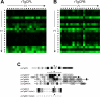
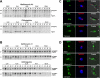
Regulated exocytosis allows the timely delivery of proteins and other macromolecules precisely when they are needed to fulfil their functions. The intracellular parasite Toxoplasma gondii has one of the most extensive regulated exocytic systems among all unicellular organisms, yet the basis of protein trafficking and proteolytic modification in this system is poorly understood. We demonstrate that a parasite cathepsin protease, TgCPL, occupies a newly recognized vacuolar compartment (VAC) that undergoes dynamic fragmentation during T. gondii replication. We also provide evidence that within the VAC or late endosome this protease mediates the proteolytic maturation of proproteins targeted to micronemes, regulated secretory organelles that deliver adhesive proteins to the parasite surface during cell invasion. Our findings suggest that processing of microneme precursors occurs within intermediate endocytic compartments within the exocytic system, indicating an extensive convergence of the endocytic and exocytic pathways in this human parasite.
Figures

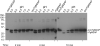


References
-
- Barrett AJ. Cathepsin G. Methods Enzymol. 1981;80(Pt C):561–565. - PubMed
-
- Botero-Kleiven S, V F., Lindh J, Richter-Dahlfors A, von Euler A, Wahlgren M. Receptor-mediated endocytosis in an apicomplexan parasite (Toxoplasma gondii). Exp Parasitol. 2001;98:134–44. - PubMed
-
- Bradley PJ, Boothroyd JC. The pro region of Toxoplasma ROP1 is a rhoptry-targeting signal. Int J Parasitol. 2001;31:1177–1186. - PubMed

