Evolutionary diversity of bile salts in reptiles and mammals, including analysis of ancient human and extinct giant ground sloth coprolites
- PMID: 20444292
- PMCID: PMC2886068
- DOI: 10.1186/1471-2148-10-133
Evolutionary diversity of bile salts in reptiles and mammals, including analysis of ancient human and extinct giant ground sloth coprolites
Abstract
Background: Bile salts are the major end-metabolites of cholesterol and are also important in lipid and protein digestion and in influencing the intestinal microflora. We greatly extend prior surveys of bile salt diversity in both reptiles and mammals, including analysis of 8,000 year old human coprolites and coprolites from the extinct Shasta ground sloth (Nothrotherium shastense).
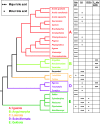
Results: While there is significant variation of bile salts across species, bile salt profiles are generally stable within families and often within orders of reptiles and mammals, and do not directly correlate with differences in diet. The variation of bile salts generally accords with current molecular phylogenies of reptiles and mammals, including more recent groupings of squamate reptiles. For mammals, the most unusual finding was that the Paenungulates (elephants, manatees, and the rock hyrax) have a very different bile salt profile from the Rufous sengi and South American aardvark, two other mammals classified with Paenungulates in the cohort Afrotheria in molecular phylogenies. Analyses of the approximately 8,000 year old human coprolites yielded a bile salt profile very similar to that found in modern human feces. Analysis of the Shasta ground sloth coprolites (approximately 12,000 years old) showed the predominant presence of glycine-conjugated bile acids, similar to analyses of bile and feces of living sloths, in addition to a complex mixture of plant sterols and stanols expected from an herbivorous diet.
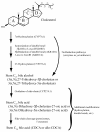
Conclusions: The bile salt synthetic pathway has become longer and more complex throughout vertebrate evolution, with some bile salt modifications only found within single groups such as marsupials. Analysis of the evolution of bile salt structures in different species provides a potentially rich model system for the evolution of a complex biochemical pathway in vertebrates. Our results also demonstrate the stability of bile salts in coprolites preserved in arid climates, suggesting that bile salt analysis may have utility in selected paleontological research.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

