Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice
- PMID: 20444418
- PMCID: PMC3020149
- DOI: 10.1016/j.cmet.2010.03.013
Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice
Abstract
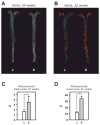
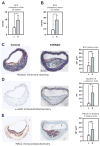
To determine whether insulin action on endothelial cells promotes or protects against atherosclerosis, we generated apolipoprotein E null mice in which the insulin receptor gene was intact or conditionally deleted in vascular endothelial cells. Insulin sensitivity, glucose tolerance, plasma lipids, and blood pressure were not different between the two groups, but atherosclerotic lesion size was more than 2-fold higher in mice lacking endothelial insulin signaling. Endothelium-dependent vasodilation was impaired and endothelial cell VCAM-1 expression was increased in these animals. Adhesion of mononuclear cells to endothelium in vivo was increased 4-fold compared with controls but reduced to below control values by a VCAM-1-blocking antibody. These results provide definitive evidence that loss of insulin signaling in endothelium, in the absence of competing systemic risk factors, accelerates atherosclerosis. Therefore, improving insulin sensitivity in the endothelium of patients with insulin resistance or type 2 diabetes may prevent cardiovascular complications.
Figures





References
-
- Abid MR, Shih SC, Otu HH, Spokes KC, Okada Y, Curiel DT, Minami T, Aird WC. A novel class of vascular endothelial growth factor-responsive genes that require forkhead activity for expression. J Biol Chem. 2006;281:35544–35553. - PubMed
-
- Aljada A, Saadeh R, Assian E, Ghanim H, Dandona P. Insulin inhibits the expression of intercellular adhesion molecule-1 by human aortic endothelial cells through stimulation of nitric oxide. J Clin Endocrinol Metab. 2000;85:2572–2575. - PubMed
-
- Atochin DN, Wang A, Liu VW, Critchlow JD, Dantas AP, Looft-Wilson R, Murata T, Salomone S, Shin HK, Ayata C, Moskowitz MA, Michel T, Sessa WC, Huang PL. The phosphorylation state of eNOS modulates vascular reactivity and outcome of cerebral ischemia in vivo. J Clin Invest. 2007;117:1961–1967. - PMC - PubMed
-
- Baumgartl J, Baudler S, Scherner M, Babaev V, Makowski L, Suttles J, McDuffie M, Tobe K, Kadowaki T, Fazio S, Kahn CR, Hotamisligil GS, Krone W, Linton M, Bruning JC. Myeloid lineage cell-restricted insulin resistance protects apolipoproteinE-deficient mice against atherosclerosis. Cell Metab. 2006;3:247–256. - PMC - PubMed
-
- Booth G, Stalker TJ, Lefer AM, Scalia R. Elevated ambient glucose induces acute inflammatory events in the microvasculature: effects of insulin. Am J Physiol Endocrinol Metab. 2001;280:E848–856. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

