Development of a two-part strategy to identify a therapeutic human bispecific antibody that inhibits IgE receptor signaling
- PMID: 20444694
- PMCID: PMC2898330
- DOI: 10.1074/jbc.M110.113910
Development of a two-part strategy to identify a therapeutic human bispecific antibody that inhibits IgE receptor signaling
Abstract
The development of bispecific antibodies as therapeutic agents for human diseases has great clinical potential, but broad application has been hindered by the difficulty of identifying bispecific antibody formats that exhibit favorable pharmacokinetic properties and ease of large-scale manufacturing. Previously, the development of an antibody technology utilizing heavy chain knobs-into-holes mutations and a single common light chain enabled the small-scale generation of human full-length bispecific antibodies. Here we have extended the technology by developing a two-part bispecific antibody discovery strategy that facilitates proof-of-concept studies and clinical candidate antibody generation. Our scheme consists of the efficient small-scale generation of bispecific antibodies lacking a common light chain and the hinge disulfides for proof-of-concept studies coupled with the identification of a common light chain bispecific antibody for large-scale production with high purity and yield. We have applied this technology to generate a bispecific antibody suitable for development as a human therapeutic. This antibody directly inhibits the activation of the high affinity IgE receptor FcepsilonRI on mast cells and basophils by cross-linking FcepsilonRI with the inhibitory receptor FcgammaRIIb, an approach that has strong therapeutic potential for asthma and other allergic diseases. Our approach for producing human bispecific full-length antibodies enables the clinical application of bispecific antibodies to a validated therapeutic pathway in asthma.
Figures


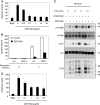
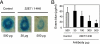
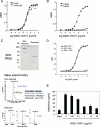
References
-
- Bargou R., Leo E., Zugmaier G., Klinger M., Goebeler M., Knop S., Noppeney R., Viardot A., Hess G., Schuler M., Einsele H., Brandl C., Wolf A., Kirchinger P., Klappers P., Schmidt M., Riethmüller G., Reinhardt C., Baeuerle P. A., Kufer P. (2008) Science 321, 974–977 - PubMed
-
- van Spriel A. B., van Ojik H. H., van De Winkel J. G. (2000) Immunol. Today 21, 391–397 - PubMed
-
- Asano R., Watanabe Y., Kawaguchi H., Fukazawa H., Nakanishi T., Umetsu M., Hayashi H., Katayose Y., Unno M., Kudo T., Kumagai I. (2007) J. Biol. Chem. 282, 27659–27665 - PubMed
-
- Carter P. (2001) J. Immunol. Methods 248, 7–15 - PubMed
-
- Kufer P., Lutterbüse R., Baeuerle P. A. (2004) Trends Biotechnol. 22, 238–244 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

