Cdk1 activity is required for mitotic activation of aurora A during G2/M transition of human cells
- PMID: 20444701
- PMCID: PMC2898447
- DOI: 10.1074/jbc.M110.141010
Cdk1 activity is required for mitotic activation of aurora A during G2/M transition of human cells
Abstract
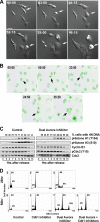
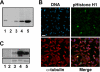
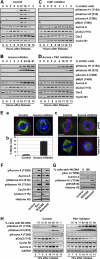
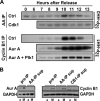
In mammalian cells entry into and progression through mitosis are regulated by multiple mitotic kinases. How mitotic kinases interact with each other and coordinately regulate mitosis remains to be fully understood. Here we employed a chemical biology approach using selective small molecule kinase inhibitors to dissect the relationship between Cdk1 and Aurora A kinases during G(2)/M transition. We find that activation of Aurora A first occurs at centrosomes at late G(2) and is required for centrosome separation independently of Cdk1 activity. Upon entry into mitosis, Aurora A then becomes fully activated downstream of Cdk1 activation. Inactivation of Aurora A or Plk1 individually during a synchronized cell cycle shows no significant effect on Cdk1 activation and entry into mitosis. However, simultaneous inactivation of both Aurora A and Plk1 markedly delays Cdk1 activation and entry into mitosis, suggesting that Aurora A and Plk1 have redundant functions in the feedback activation of Cdk1. Together, our data suggest that Cdk1, Aurora A, and Plk1 mitotic kinases participate in a feedback activation loop and that activation of Cdk1 initiates the feedback loop activity, leading to rapid and timely entry into mitosis in human cells. In addition, live cell imaging reveals that the nuclear cycle of cells becomes uncoupled from cytokinesis upon inactivation of both Aurora A and Aurora B kinases and continues to oscillate in a Cdk1-dependent manner in the absence of cytokinesis, resulting in multinucleated, polyploidy cells.
Figures
Similar articles
-
WAC Promotes Polo-like Kinase 1 Activation for Timely Mitotic Entry.Cell Rep. 2018 Jul 17;24(3):546-556. doi: 10.1016/j.celrep.2018.06.087. Cell Rep. 2018. PMID: 30021153
-
Inactivation of Rho GTPases with Clostridium difficile toxin B impairs centrosomal activation of Aurora-A in G2/M transition of HeLa cells.Mol Biol Cell. 2007 Oct;18(10):3752-63. doi: 10.1091/mbc.e07-03-0281. Epub 2007 Jul 18. Mol Biol Cell. 2007. PMID: 17634283 Free PMC article.
-
FRET-Based Sorting of Live Cells Reveals Shifted Balance between PLK1 and CDK1 Activities During Checkpoint Recovery.Cells. 2020 Sep 19;9(9):2126. doi: 10.3390/cells9092126. Cells. 2020. PMID: 32961751 Free PMC article.
-
Aurora-PLK1 cascades as key signaling modules in the regulation of mitosis.Sci Signal. 2018 Aug 14;11(543):eaar4195. doi: 10.1126/scisignal.aar4195. Sci Signal. 2018. PMID: 30108183 Review.
-
Checking out the centrosome.Cell Cycle. 2004 Nov;3(11):1390-3. doi: 10.4161/cc.3.11.1252. Epub 2004 Nov 22. Cell Cycle. 2004. PMID: 15483402 Review.
Cited by
-
A functional cooperativity between Aurora A kinase and LIM kinase1: implication in the mitotic process.Cell Cycle. 2012 Jan 15;11(2):296-309. doi: 10.4161/cc.11.2.18734. Epub 2012 Jan 15. Cell Cycle. 2012. PMID: 22214762 Free PMC article.
-
The LC3B FRET biosensor monitors the modes of action of ATG4B during autophagy in living cells.Autophagy. 2023 Aug;19(8):2275-2295. doi: 10.1080/15548627.2023.2179845. Epub 2023 Feb 22. Autophagy. 2023. PMID: 36814061 Free PMC article.
-
Induction of a Spindle-Assembly-Competent M Phase in Xenopus Egg Extracts.Curr Biol. 2019 Apr 22;29(8):1273-1285.e5. doi: 10.1016/j.cub.2019.02.061. Epub 2019 Mar 28. Curr Biol. 2019. PMID: 30930041 Free PMC article.
-
The apoptotic mechanism of action of the sphingosine kinase 1 selective inhibitor SKI-178 in human acute myeloid leukemia cell lines.J Pharmacol Exp Ther. 2015 Mar;352(3):494-508. doi: 10.1124/jpet.114.219659. Epub 2015 Jan 6. J Pharmacol Exp Ther. 2015. PMID: 25563902 Free PMC article.
-
Spatial-temporal model for silencing of the mitotic spindle assembly checkpoint.Nat Commun. 2014 Sep 12;5:4795. doi: 10.1038/ncomms5795. Nat Commun. 2014. PMID: 25216458 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

