Tracking mutant huntingtin aggregation kinetics in cells reveals three major populations that include an invariant oligomer pool
- PMID: 20444706
- PMCID: PMC2898425
- DOI: 10.1074/jbc.M109.084434
Tracking mutant huntingtin aggregation kinetics in cells reveals three major populations that include an invariant oligomer pool
Abstract
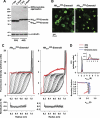
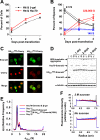
Huntington disease is caused by expanded polyglutamine sequences in huntingtin, which procures its aggregation into intracellular inclusion bodies (IBs). Aggregate intermediates, such as soluble oligomers, are predicted to be toxic to cells, yet because of a lack of quantitative methods, the kinetics of aggregation in cells remains poorly understood. We used sedimentation velocity analysis to define and compare the heterogeneity and flux of purified huntingtin with huntingtin expressed in mammalian cells under non-denaturing conditions. Non-pathogenic huntingtin remained as hydrodynamically elongated monomers in vitro and in cells. Purified polyglutamine-expanded pathogenic huntingtin formed elongated monomers (2.4 S) that evolved into a heterogeneous aggregate population of increasing size over time (100-6,000 S). However, in cells, mutant huntingtin formed three major populations: monomers (2.3 S), oligomers (mode s(20,w) = 140 S) and IBs (mode s(20,w) = 320,000 S). Strikingly, the oligomers did not change in size heterogeneity or in their proportion of total huntingtin over 3 days despite continued monomer conversion to IBs, suggesting that oligomers are rate-limiting intermediates to IB formation. We also determined how a chaperone known to modulate huntingtin toxicity, Hsc70, influences in-cell huntingtin partitioning. Hsc70 decreased the pool of 140 S oligomers but increased the overall flux of monomers to IBs, suggesting that Hsc70 reduces toxicity by facilitating transfer of oligomers into IBs. Together, our data suggest that huntingtin aggregation is streamlined in cells and is consistent with the 140 S oligomers, which remain invariant over time, as a constant source of toxicity to cells irrespective of total load of insoluble aggregates.
Figures



References
-
- Chiti F., Dobson C. M. (2006) Annu. Rev. Biochem. 75, 333–366 - PubMed
-
- Scherzinger E., Lurz R., Turmaine M., Mangiarini L., Hollenbach B., Hasenbank R., Bates G. P., Davies S. W., Lehrach H., Wanker E. E. (1997) Cell 90, 549–558 - PubMed
-
- Arrasate M., Mitra S., Schweitzer E. S., Segal M. R., Finkbeiner S. (2004) Nature 431, 805–810 - PubMed
-
- Takahashi Y., Okamoto Y., Popiel H. A., Fujikake N., Toda T., Kinjo M., Nagai Y. (2007) J. Biol. Chem. 282, 24039–24048 - PubMed
-
- Takahashi T., Kikuchi S., Katada S., Nagai Y., Nishizawa M., Onodera O. (2007) Hum. Mol. Genet. 17, 345–346 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

